Verteporfin (Synonyms: CL 318952;Visudyne) |
| Catalog No.GC14482 |
Verteporfin is a potent inhibitor of PD-L1 expression used for treatment in the age-related macular degeneration , port-wine stain birthmarks and cancer.
Products are for research use only. Not for human use. We do not sell to patients.
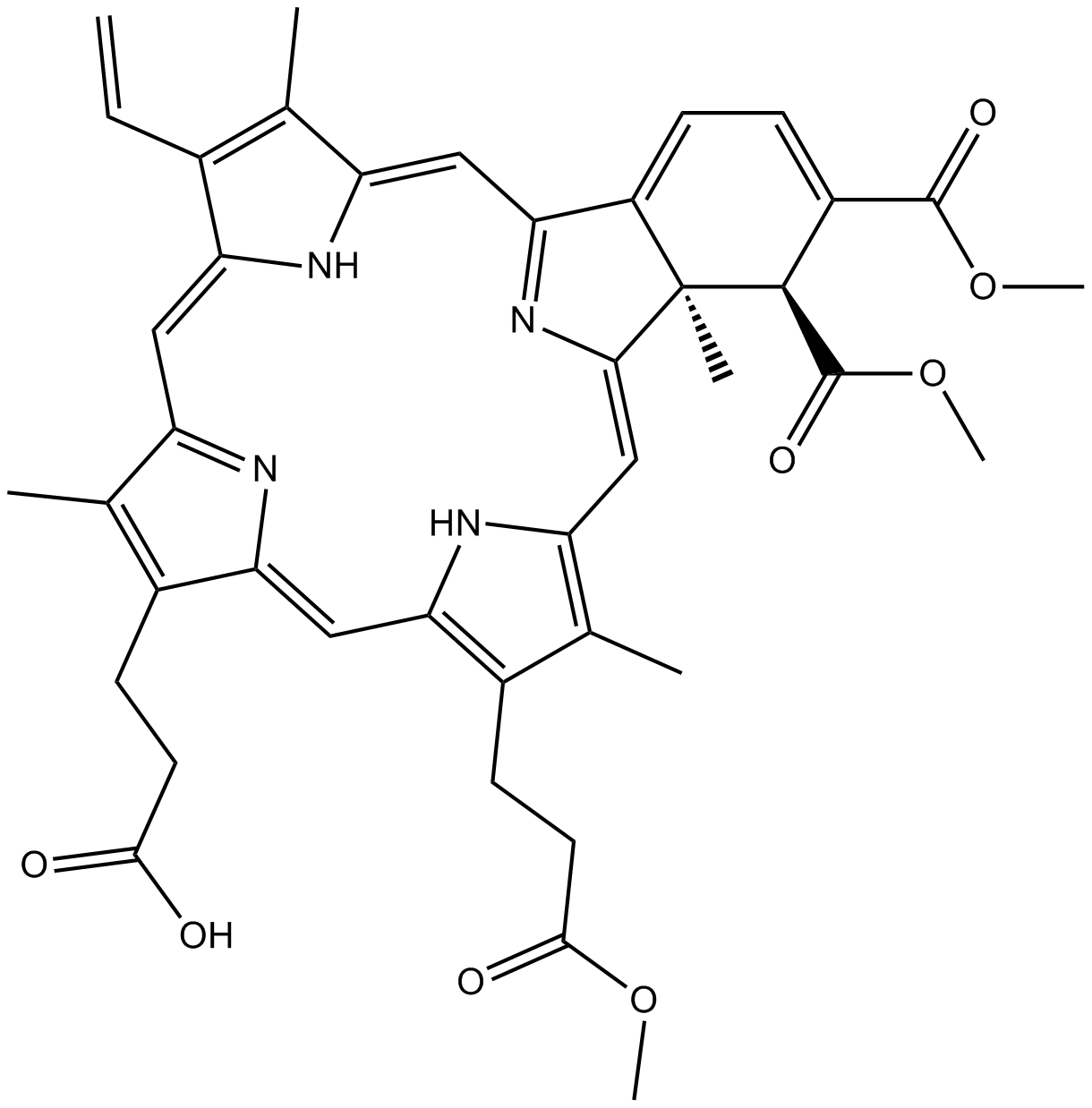
Cas No.: 129497-78-5
Sample solution is provided at 25 µL, 10mM.
Verteporfin is a potent inhibitor of PD-L1 expression used for treatment in the age-related macular degeneration , port-wine stain birthmarks and cancer.[1]
In vitro experiment it shown that at 1μM versus 0.5 μM concentrations of verteporfin, it was sufficient to decrease PD-L1 expression in ATG5 depleted cells.[1] In vitro, at very low concentrations of verteporfin (0.1–0.2 μm) , verteporfin induced significant cell death in GSCs. However, 0.5 μm verteporfin had no effect on the cell death in differentiated GSCs, IMR90, rat NSCs or mouse astrocytes.[2] In vitro efficacy test it indicated treatment with 4, 8 and 12 μM verteporfin decreased significantly the expression levels of YAP, AXL and CYR61 mRNA in MCF-7, BT-474 and BT-549 cells. And verteporfin also downregulated the mRNA expression of CTGF in BT-474 and BT-549 cells.[3] Treatment with 0.5, 1, 2, and 5 μM verteporfin inhibited the viability of HeLa cells and had no obvious effect on H8 cells.[4]
In vivo study it demonstrated that treatment with 2 mg.kg-1 free verteporfin induced severe phototoxic adverse effects leading to the death of 5 out of 8 mice. However, mice were treated 8 mg.kg-1 nanostructured lipid carriers-verteporfin intravenously laser light exposure of tumors significantly inhibited tumor growth without visible toxicity.[5] In vivo, 2 mg/kg verteporfin infusion alone resulted in nitric oxide and malonyldialdehyde level increments in the retina.[6]
References:
[1].Liang J, et al. Verteporfin Inhibits PD-L1 through Autophagy and the STAT1-IRF1-TRIM28 Signaling Axis, Exerting Antitumor Efficacy. Cancer Immunol Res. 2020 Jul;8(7):952-965. Kuramoto K, Yamamoto M, Suzuki S, Sanomachi T, Togashi K, Seino S, Kitanaka C, Okada M.
[2].Kuramoto K, et al. Verteporfin inhibits oxidative phosphorylation and induces cell death specifically in glioma stem cells. FEBS J. 2020 May;287(10):2023-2036.
[3].Wei C, Li X. Verteporfin inhibits cell proliferation and induces apoptosis in different subtypes of breast cancer cell lines without light activation. BMC Cancer. 2020 Oct 29;20(1):1042.
[4].Yin L, Chen G. Verteporfin Promotes the Apoptosis and Inhibits the Proliferation, Migration, and Invasion of Cervical Cancer Cells by Downregulating SULT2B1 Expression. Med Sci Monit. 2020 Oct 20;26:e926780.
[5].Michy T, et al. Verteporfin-Loaded Lipid Nanoparticles Improve Ovarian Cancer Photodynamic Therapy In Vitro and In Vivo. Cancers (Basel). 2019 Nov 8;11(11):1760.
[6].Turkuoglu P, et al. Retinal nitric oxide and malonyldialdehyde levels following photodynamic therapy. Indian J Ophthalmol. 2011 Jan-Feb;59(1):5-8.
Average Rating: 5 (Based on Reviews and 24 reference(s) in Google Scholar.)
GLPBIO products are for RESEARCH USE ONLY. Please make sure your review or question is research based.
Required fields are marked with *




