A 83-01 |
| Catalog No.GC10166 |
A TGF-β type I receptor inhibitor
Products are for research use only. Not for human use. We do not sell to patients.
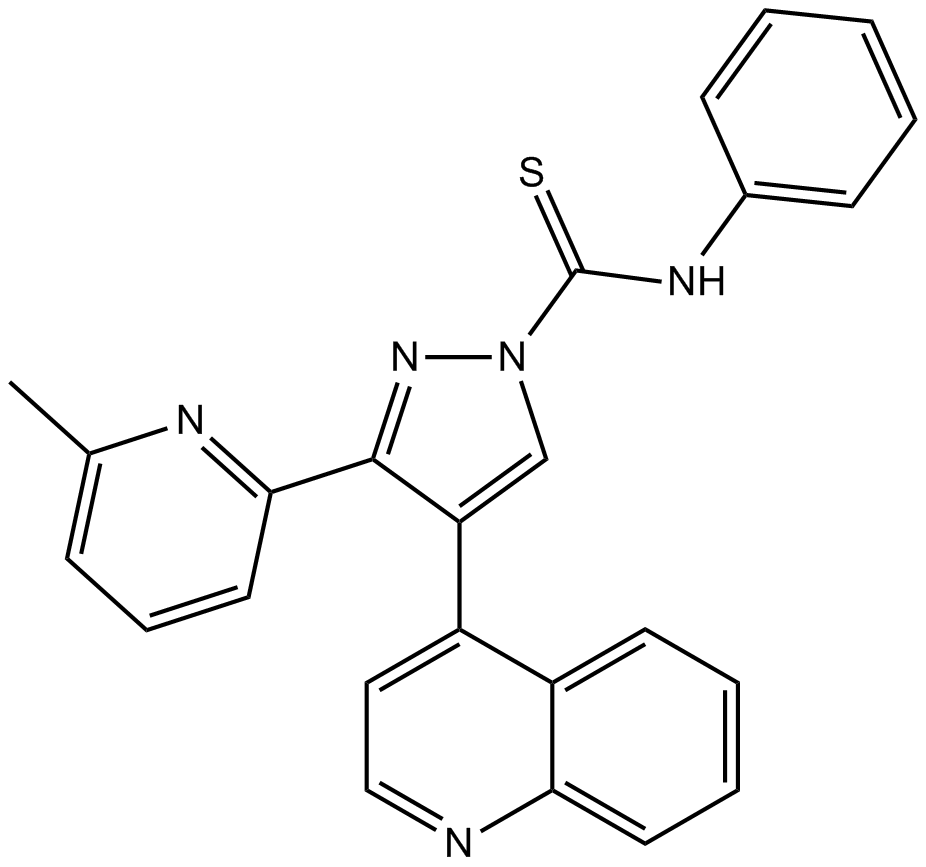
Cas No.: 909910-43-6
Sample solution is provided at 25 µL, 10mM.
A 83-01 is a potent inhibitor of TGF-β type I receptor ALK5 kinase, type I nodal receptor ALK4 and type I nodal receptor ALK7, with IC50 s of 12 nM,45 nM and 7.5 nM against the transcription induced by ALK5, ALK4 and ALK7, respectively [1].
A 83-01 reduces the level of ALK-5-induced transcription in Mv1Lu cells, also blocks the ALK4-TD and ALK7-TD induced transcription R4-2 cells, and weakly suppresses that induced by constitutively active ALK-6, ALK-2, ALK-3, and ALK-1. A 83-01 (0.03-10 μM) potently prevents the growth-inhibitory effects of TGF-β, and completely inhibits the effect at 3 μM. A 83-01 (1-10 μM) inhibits TGF-β-induced Smad activation in HaCaT cells [1]. A 83-01 (1 μM) decreases cell motility, adhesion and invasion increased by TGF-β1 in HM-1 cells, but does not change cell proliferation [2]. A-83-01-treated retinal pigment epithelium (RPE) failed to differentiate after 7 passages (P7). Exogenous expression of MYCN and OTX2 in conjunction with A 83-01 restored P7-RPE differentiation to a status similar to minimally passaged RPE [5].
When co-administrated F-SL with A 83-01. Intraperitoneally injected A 83-01-induced alterations in the cancer-associated neovasculature were examined by magnetic resonance imaging (MRI) and histological analysis. The targeting efficacy of single intravenous injections of F-SL combined with A 83-01 was evaluated by measurement of the biodistribution and the antitumor effect in mice bearing murine lung carcinoma M109. A 83-01 temporarily changed the tumor vasculature around 3 h post injection. A 83-01 induced 1.7-fold higher drug accumulation of F-SL in the tumor than liposome alone at 24 h post injection. Moreover F-SL co-administrated with A 83-01 showed significantly greater antitumor activity than F-SL alone [3]. A 83-01 treatment significantly increased the number of Nkx2.5(+) cardiomyoblasts at baseline and after myocardial injury, resulting in an increase in newly formed cardiomyocytes [6]. Using transgenic Nkx2.5 enhancer-green fluorescent protein (GFP) reporter mice, and isolated cardiac progenitor cells (CPC). A 83-01 was found to induce proliferation mainly through increasing Birc5 expression in the MEK/ERK-dependent pathway [7]. Treatment of rat dermal fibroblast with A 83-01 inhibited transforming growth factor-β1 (TGF-β1)-dependent induction of α-SMA and collagen type I [4].
References:
[1]: Tojo M, Hamashima Y, et,al. The ALK-5 inhibitor A-83-01 inhibits Smad signaling and epithelial-to-mesenchymal transition by transforming growth factor-beta. Cancer Sci. 2005 Nov;96(11):791-800. doi: 10.1111/j.1349-7006.2005.00103.x. PMID: 16271073.
[2]: Yamamura S, Matsumura N, et,al. The activated transforming growth factor-beta signaling pathway in peritoneal metastases is a potential therapeutic target in ovarian cancer. Int J Cancer. 2012 Jan 1;130(1):20-8. doi: 10.1002/ijc.25961. Epub 2011 Apr 18. PMID: 21503873.
[3]: Taniguchi Y, Kawano K, et,al. Enhanced antitumor efficacy of folate-linked liposomal doxorubicin with TGF-β type I receptor inhibitor. Cancer Sci. 2010 Oct;101(10):2207-13. doi: 10.1111/j.1349-7006.2010.01646.x. Epub 2010 Jul 1. PMID: 20608940.
[4]: Sun X, Kim YH, et,al. Topical application of ALK5 inhibitor A-83-01 reduces burn wound contraction in rats by suppressing myofibroblast population. Biosci Biotechnol Biochem. 2014;78(11):1805-12. doi: 10.1080/09168451.2014.932666. Epub 2014 Jul 10. PMID: 25351330.
[5]:Shih YH, Radeke MJ, et,al. Restoration of Mesenchymal RPE by Transcription Factor-Mediated Reprogramming. Invest Ophthalmol Vis Sci. 2017 Jan 1;58(1):430-441. doi: 10.1167/iovs.16-20018. PMID: 28118667.
[6]: Chen WP, Liu YH, et,al. Pharmacological inhibition of TGFβ receptor improves Nkx2.5 cardiomyoblast-mediated regeneration. Cardiovasc Res. 2015 Jan 1;105(1):44-54. doi: 10.1093/cvr/cvu229. Epub 2014 Oct 31. PMID: 25362681; PMCID: PMC4342671.
[7]: Ho YS, Tsai WH,et,al. Cardioprotective Actions of TGFβRI Inhibition Through Stimulating Autocrine/Paracrine of Survivin and Inhibiting Wnt in Cardiac Progenitors. Stem Cells. 2016 Feb;34(2):445-55. doi: 10.1002/stem.2216. Epub 2015 Oct 10. PMID: 26418219.
Average Rating: 5 (Based on Reviews and 30 reference(s) in Google Scholar.)
GLPBIO products are for RESEARCH USE ONLY. Please make sure your review or question is research based.
Required fields are marked with *




















