BPTES |
| Catalog No.GC13958 |
A GLS inhibitor
Products are for research use only. Not for human use. We do not sell to patients.
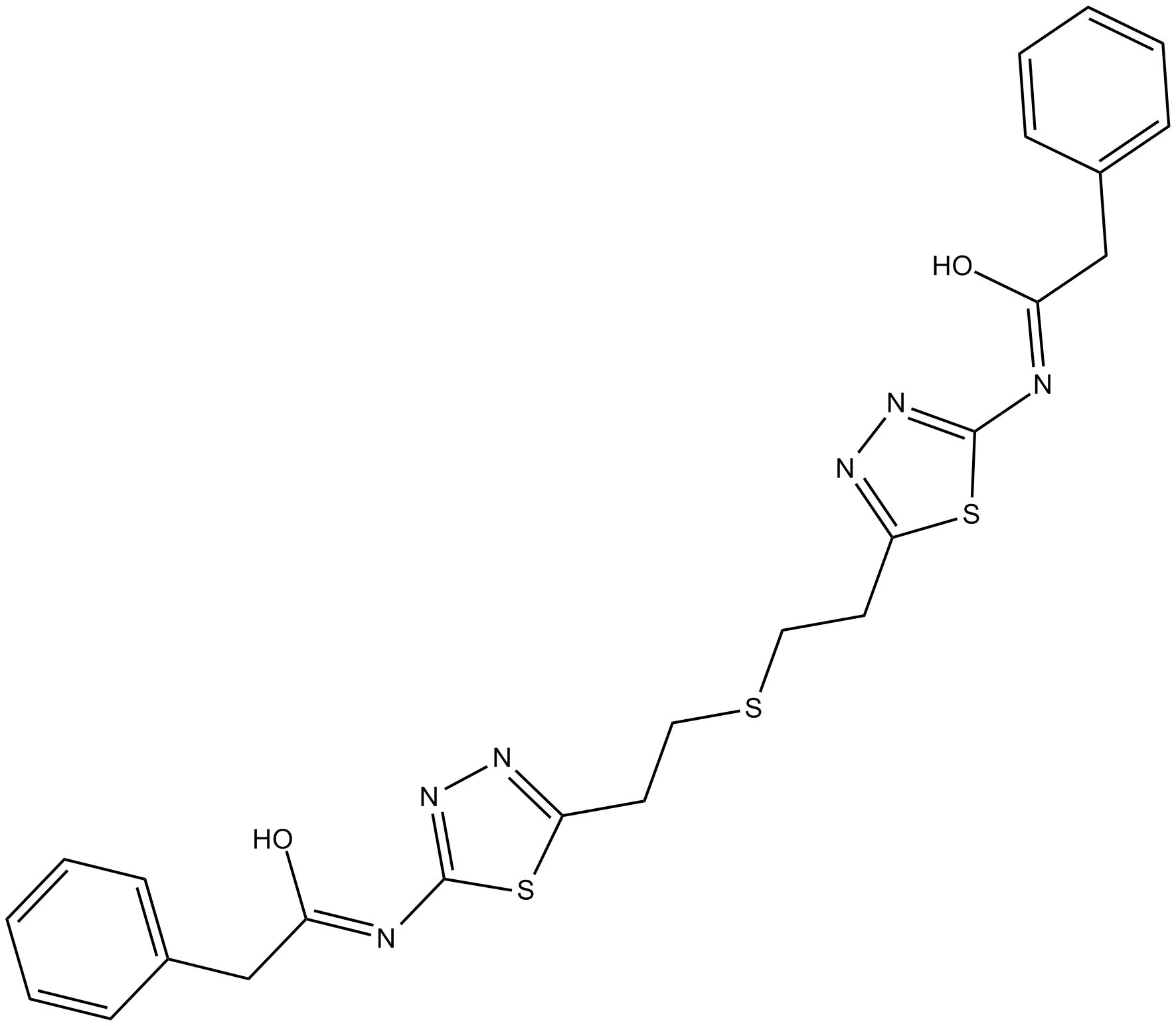
Cas No.: 314045-39-1
Sample solution is provided at 25 µL, 10mM.
BPTES is a potent and selective kidney-type glutaminase (GLS) inhibitor [1], with a Ki value of approx. 3 μM [2].
Glutaminase hydrolyzes glutamine into ammonia and glutamate. In mammalian tissues, two glutaminase isoforms derived from structurally related but distinct genes, are expressed. GLS is widely distributed in extra-hepatic tissues. Liver-type glutaminase (GLS2) is primarily found in adult liver. GLS is critical in glutaminolysis for many proliferating cells, especially malignant cells with rapid growth [1].
Cell lines with mutations in isocitrate dehydrogenase 1 and 2 (IDH1/2) were used. In all IDH1-mutant AML cells, compared with DMSO, exposure to 20 µmol/L BPTES reduced the cell growth by approximately 50% on day 4. 20 µmol/L BPTES was not significantly different from 40 µmol/L BPTES in the reduction effect. Treatment without drug was not significantly different from treatment with DMSO in the growth of cells. BPTES did not significantly affect the cell growth of wild type AML cells [3]. In tumor cells, BPTES inhibited the conversion of glutamine into glutamate [4].
Glutamate is a substrate of GPT in the transamination of pyruvate to alanine. Compared with controls, BPTES treatment reduced the pyruvate-to-alanine conversion in animals. In replicated experiments, BPTES significantly reduce the alanine-to-pyruvate (Ala/Pyr) flux ratio [4].
References:
[1]. Shukla K, Ferraris DV, Thomas AG, et al. Design, synthesis, and pharmacological evaluation of bis-2-(5-phenylacetamido-1, 2, 4-thiadiazol-2-yl) ethyl sulfide 3 (BPTES) analogs as glutaminase inhibitors. Journal of medicinal chemistry, 2012, 55(23): 10551-10563.
[2]. Robinson MM, Mcbryant SJ, Tsukamoto T, et al. Novel mechanism of inhibition of rat kidney-type glutaminase by bis-2-(5-phenylacetamido-1, 2, 4-thiadiazol-2-yl) ethyl sulfide (BPTES). Biochemical Journal, 2007, 406(3): 407-414.
[3]. Emadi A, Jun SA, Tsukamoto T, et al. Inhibition of glutaminase selectively suppresses the growth of primary acute myeloid leukemia cells with IDH mutations. Experimental hematology, 2014, 42(4): 247-251.
[4]. Dutta P, Le A, Vander Jagt DL, et al. Evaluation of LDH-A and glutaminase inhibition in vivo by hyperpolarized 13C-pyruvate magnetic resonance spectroscopy of tumors. Cancer research, 2013, 73(14): 4190-4195.
Average Rating: 5 (Based on Reviews and 20 reference(s) in Google Scholar.)
GLPBIO products are for RESEARCH USE ONLY. Please make sure your review or question is research based.
Required fields are marked with *




















