Fulvestrant (ICI 182,780) (Synonyms: ICI 182780) |
| Catalog No.GC18000 |
Fulvestrant is a selective estrogen receptor (ER) antagonist. It binds, blocks and degrades estrogen receptor, then inhibits estrogen receptor(ER)-mediated transcriptional activity with an IC50 of 9.4 nM .
Products are for research use only. Not for human use. We do not sell to patients.
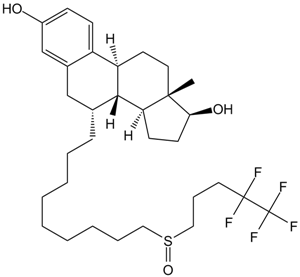
Cas No.: 129453-61-8
Sample solution is provided at 25 µL, 10mM.
Fulvestrant is a selective estrogen receptor (ER) antagonist. It binds, blocks and degrades estrogen receptor, then inhibits estrogen receptor(ER)-mediated transcriptional activity with an IC50 of 9.4 nM [1-3].
Fulvestrant(3 μM ;1 month) increases the sensitivity of H1975 NSCLC cells to gefitinib[4].Fulvestrant(1-10 µM;72 h) strongly sensitized doxorubicin-induced cytotoxicity in MDR cell lines[5].
Fulvestrant(5 mg/mouse; s.c.; twice per week) significantly inhibited macrophage and neutrophil infiltration in mice cancer model. Fulvestrant decreases ER+ breast cancer growth in the presence of physiologic levels of estradiol in human breast cancer in nude mice[6]. Fulvestrant (5mg/mouse; twice weekly; s.c.) in combination with tamoxifen enhanced tumor regression[7].
References:
[1]. Vergote I, Robertson JF. Fulvestrant is an effective and well-tolerated endocrine therapy for postmenopausal women with advanced breast cancer: results from clinical trials. Br J Cancer. 2004 Mar;90 Suppl 1(Suppl 1):S11-4. doi: 10.1038/sj.bjc.6601631. PMID: 15094759; PMCID: PMC2750769.
[2]. Osborne CK, Wakeling A, et,al. Fulvestrant: an oestrogen receptor antagonist with a novel mechanism of action. Br J Cancer. 2004 Mar;90 Suppl 1(Suppl 1):S2-6. doi: 10.1038/sj.bjc.6601629. PMID: 15094757; PMCID: PMC2750773.
[3]. Dowsett M, Nicholson RI, et,al. Biological characteristics of the pure antiestrogen fulvestrant: overcoming endocrine resistance. Breast Cancer Res Treat. 2005;93 Suppl 1:S11-8. doi: 10.1007/s10549-005-9037-3. PMID: 16247595.
[4]. Shen H, Liu J, et,al. Fulvestrant increases gefitinib sensitivity in non-small cell lung cancer cells by upregulating let-7c expression. Biomed Pharmacother. 2014 Apr;68(3):307-13. doi: 10.1016/j.biopha.2013.10.007. Epub 2013 Nov 7. PMID: 24268810.
[5]. Huang Y, Jiang D, et,al. Fulvestrant reverses doxorubicin resistance in multidrug-resistant breast cell lines independent of estrogen receptor expression. Oncol Rep. 2017 Feb;37(2):705-712. doi: 10.3892/or.2016.5315. Epub 2016 Dec 14. PMID: 28000875; PMCID: PMC5355712.
[6]. Abrahamsson A, Rodriguez GV, et,al. Fulvestrant-Mediated Attenuation of the Innate Immune Response Decreases ER+ Breast Cancer Growth In Vivo More Effectively than Tamoxifen. Cancer Res. 2020 Oct 15;80(20):4487-4499. doi: 10.1158/0008-5472.CAN-20-1705. Epub 2020 Aug 27. PMID: 32855207.
[7]. Mishra AK, Abrahamsson A, et,al. Fulvestrant inhibits growth of triple negative breast cancer and synergizes with tamoxifen in ERα positive breast cancer by up-regulation of ERβ. Oncotarget. 2016 Aug 30;7(35):56876-56888. doi: 10.18632/oncotarget.10871. PMID: 27486755; PMCID: PMC5302959.
Average Rating: 5 (Based on Reviews and 22 reference(s) in Google Scholar.)
GLPBIO products are for RESEARCH USE ONLY. Please make sure your review or question is research based.
Required fields are marked with *







