Cycloheximide (Synonyms: Naramycin A; Actidione; 3-[2-(3,5-Dimethyl-2-oxocyclohexyl)-2-hydroxyethyl]glutarimide) |
| Catalog No.GC17198 |
Cycloheximide is an antibiotic that inhibits protein synthesis at the translation level, acting exclusively on cytoplasmic (80s) ribosomes of eukaryotes.
Products are for research use only. Not for human use. We do not sell to patients.
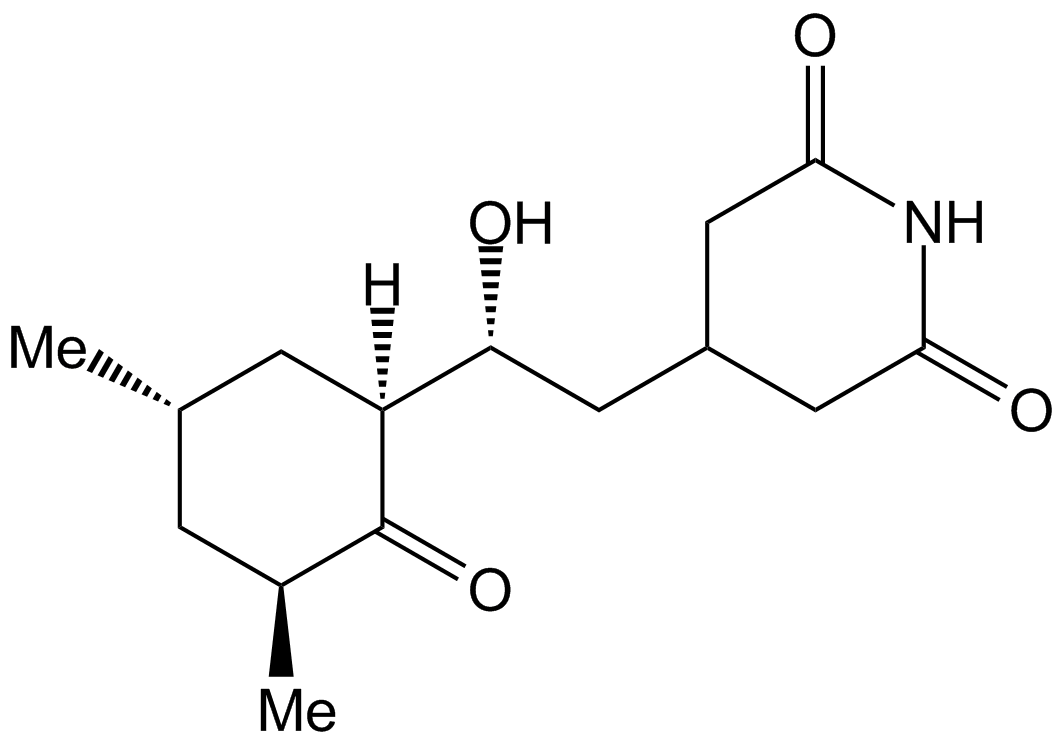
Cas No.: 66-81-9
Sample solution is provided at 25 µL, 10mM.
- Adv Sci (2023): 2303113.PMID:37877615
- Oncogene (2023): 1-13.PMID:38040806
- Cell Signal (2024):111064.PMID:38266744
- Drug Resist Update (2024):101063.PMID:38335844
- J Mol Endocrinol 72.3 (2024).PMID:38261314
- J Pharm Anal 14.1 (2024):69-85.PMID:38352950
- J Biol Chem (2024):107139.PMID:38447792
- J Biol Chem 300.4 (2024).PMID:38447792
Cycloheximide is an antibiotic that inhibits protein synthesis at the translation level, acting exclusively on cytoplasmic (80s) ribosomes of eukaryotes. Cycloheximide affected all the energy-dependent stages in the protein-synthesizing process. However, the initiation seems the most sensitive. Cycloheximide also affects respiration, ion uptake, amino acid biosynthesis, and DNA and RNA synthesis, effects that are probably secondary to its effect on protein synthesis.[1]
In vitro study indicated that Cycloheximide at 1 μM inhibited [3H]leucine incorporation into both cellular and secreted proteins by at least 86%, without having deleterious effects on membrane integrity as indicated by trypan blue uptake and lactate dehydrogenase release. Larger size class polysomes (7+) were increased by Cycloheximide treatment and remained increased during recovery. [2]
In vivo analysis indicated that Cycloheximide produced initial hyperactivity. This initial hyperactivity was apparent within 3 minutes after injection of the Cycloheximide. Cycloheximide affects activity by acting on the brain, and this is unrelated to its inhibition of protein synthesis. In addition, Cycloheximide’s effects on activity did not appear to be responsible for its amnesic action. However, Cycloheximide might have some other property, unrelated to inhibition of cerebral protein synthesis, that is responsible for its amnesic effect.[3]
References:
[1]. Marcos R, et al. Effect of Cycloheximide on different stages of Drosophila melanogaster. Toxicol Lett. 1982 Sep;13(1-2):105-12.
[2].Helinek TG, et al. Initial inhibition and recovery of protein synthesis in cycloheximide-treated hepatocytes. Biochem Pharmacol. 1982 Apr 1;31(7):1219-25.
[3]. Segal DS, et al. Cycloheximide: its effects on activity are dissociable from its effects on memory. Science. 1971 Apr 2;172(3978):82-4.
Average Rating: 5 (Based on Reviews and 30 reference(s) in Google Scholar.)
GLPBIO products are for RESEARCH USE ONLY. Please make sure your review or question is research based.
Required fields are marked with *




