TBTA (Synonyms: Tris(benzyltriazolylmethyl)amine) |
| Catalog No.GC45003 |
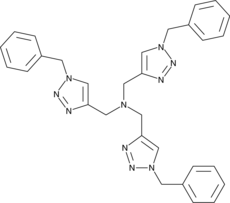
TBTA is a ligand that acts as a biochemical tool for the tagging of proteins and enzymes. TBTA is also an effective catalyst for click “cyclo” addition.
Products are for research use only. Not for human use. We do not sell to patients.
Cas No.: 510758-28-8
Sample solution is provided at 25 µL, 10mM.
TBTA is a ligand that can be used as a biochemical tool to label proteins and enzymes. TBTA is a tertiary amine containing a 1,2,3-triazole group. When used as a ligand, TBTA complexes with copper(I) and promotes catalysis by stabilizing the copper(I)-oxidation state to accelerate azide-alkyne cycloaddition reactions, as used in click chemistry [1].
References:
[1] Alireza Movahedi, et al. One-pot synthesis of TBTA-functionalized coordinating polymers. Reactive and Functional Polymers.2014. 82:1-8.
Average Rating: 5 (Based on Reviews and 18 reference(s) in Google Scholar.)
GLPBIO products are for RESEARCH USE ONLY. Please make sure your review or question is research based.
Required fields are marked with *




