BMPO (Synonyms: BocMPO) |
| Catalog No.GC41397 |
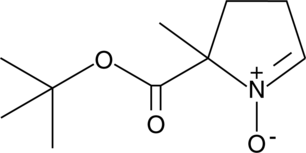
BMPO is a cyclic nitrone spin trap agent, it is a water-soluble white solid which makes BMPO purification easier than other spin trap agents.
Products are for research use only. Not for human use. We do not sell to patients.
Cas No.: 387334-31-8
Sample solution is provided at 25 µL, 10mM.
BMPO is a cyclic nitrone spin trap agent, it is a water-soluble white solid which makes BMPO purification easier than other spin trap agents. Even after prolonged storage at 220°C, there was no artificial signal formation from aqueous solutions containing BMPO (25–100 mM). BMPO offers several advantages over the existing spin traps in the detection and characterization of thiyl radicals, hydroxyl radicals, and superoxide anions in biological systems. One of the perceived advantages of BMPO is that the BMPO/•OOH adduct does not readily decay nonenzymatically to the BMPO/•OH adduct. [1]
The in vitro experiment took advantage of Rabbit aortic segments, which were treated with ADR and incubation with 0.1 M BMPO, a novel solid superoxide spin trap. Results showed that BMPO-hydroxyl radical adduct was detected in supernatants upon 10-min incubation of aortic segment with incremental doses of ADR.[2] Time-dependent changes in the ESR spectra of superoxide adducts of BMPO was generated in a xanthine/xanthine oxidase incubation system. BMPO superoxide adducts did not decay to the corresponding hydroxyl adducts. Results also indicate that the BMPO superoxide adduct is persistent. The decay kinetics of BMPO/•OOH also demonstrate this feature. In this system, the ESR spectrum of the BMPO/•OOH adduct could be detected even up to 35 min. Although BMPO/•OOH is intrinsically more stable, it is likely to be enzymatically reduced to BMPO/•OH in biological systems.[1]
References:
[1]. Zhao H, Joseph J, Zhang H, Karoui H, Kalyanaraman B. Synthesis and biochemical applications of a solid cyclic nitrone spin trap: a relatively superior trap for detecting superoxide anions and glutathiyl radicals. Free Radic Biol Med. 2001 Sep 1;31(5):599-606.
[2]. Duquaine D, Hirsch GA, Chakrabarti A, Han Z, Kehrer C, Brook R, Joseph J, Schott A, Kalyanaraman B, Vasquez-Vivar J, Rajagopalan S. Rapid-onset endothelial dysfunction with adriamycin: evidence for a dysfunctional nitric oxide synthase. Vasc Med. 2003 May;8(2):101-7.
Average Rating: 5 (Based on Reviews and 18 reference(s) in Google Scholar.)
GLPBIO products are for RESEARCH USE ONLY. Please make sure your review or question is research based.
Required fields are marked with *




