Estrone (Synonyms: E1) |
| Catalog No.GC14385 |
Estrogenic hormone
Products are for research use only. Not for human use. We do not sell to patients.
Cas No.: 53-16-7
Sample solution is provided at 25 µL, 10mM.
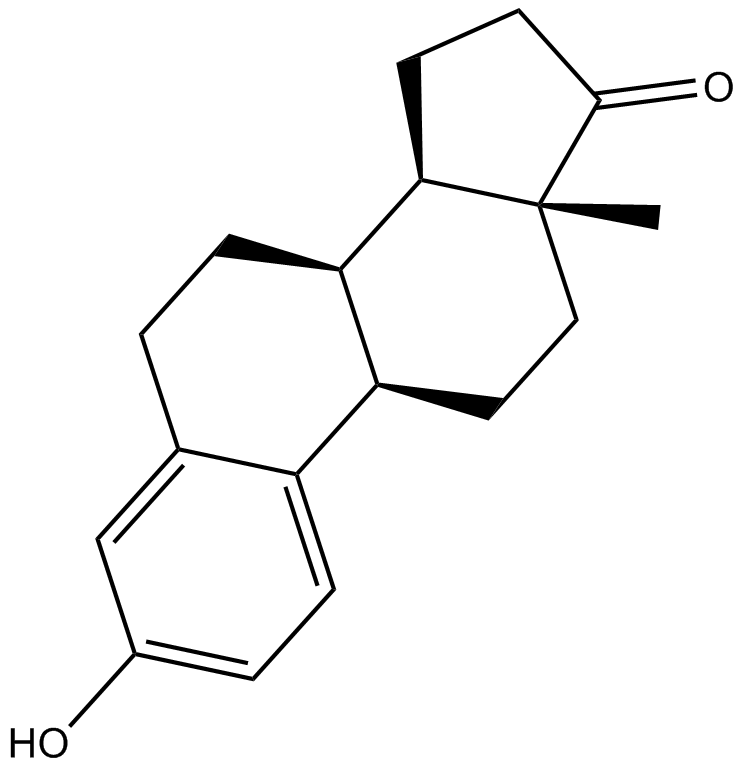
Estrone is an estrogenic hormone.Target: Estrogen Receptor/ERREstrone (E1) is an estrogenic hormone secreted by the ovary as well as adipose tissue with the chemical name of 3-hydroxyestra-1,3,5(10)-triene-17-one and the chemical formula C18H22O2. Estrone is one of several natural estrogens, which also include estriol and estradiol. Estrone is the least abundant of the three hormones; estradiol is present almost always in the reproductive female body, and estriol is abundant primarily during pregnancy. Estrone is relevant to health and disease states because of its conversion to estrone sulfate, a long-lived derivative. Estrone sulfate acts as a reservoir that can be converted as needed to the more active estradiol. It is the predominant estrogen in postmenopausal women [1, 2].
References:
[1]. Hoffmann, B., et al., [Profiles of estrone, estrone sulfate and progesterone in donkey (Equus asinus) mares during pregnancy]. Tierarztl Prax Ausg G Grosstiere Nutztiere, 2014. 42(1): p. 32-9.
[2]. Oneson, I.B. and S.L. Cohen, The nature of the conjugated estrone in human pregnancy urine. Endocrinology, 1952. 51(3): p. 173-82.
Average Rating: 5 (Based on Reviews and 12 reference(s) in Google Scholar.)
GLPBIO products are for RESEARCH USE ONLY. Please make sure your review or question is research based.
Required fields are marked with *




















