Proteases
Proteases is a general term for a class of enzymes that hydrolyze protein peptide chains. According to the way they degrade polypeptides, they are divided into two categories: endopeptidases and telopeptidases. The former can cut the large molecular weight polypeptide chain from the middle to form prions and peptones with smaller molecular weights; the latter can be divided into carboxypeptidase and aminopeptidase, which respectively remove the peptide from the free carboxyl terminus or free amino terminus of the polypeptide one by one. Chain hydrolysis produces amino acids.
A general term for a class of enzymes that hydrolyze peptide bonds in proteins. According to the way they hydrolyze polypeptides, they can be divided into endopeptidases and exopeptidases. Endopeptidase cleaves the interior of the protein molecule to form smaller molecular weight peptones and peptones. Exopeptidase hydrolyzes peptide bonds one by one from the end of the free amino group or carboxyl group of protein molecules, and frees amino acids, the former is aminopeptidase and the latter is carboxypeptidase. Proteases can be classified into serine proteases, sulfhydryl proteases, metalloproteases and aspartic proteases according to their active centers and optimum pH. According to the optimum pH value of its reaction, it is divided into acidic protease, neutral protease and alkaline protease. The proteases used in industrial production are mainly endopeptidases.
Proteases are widely found in animal offal, plant stems and leaves, fruits and microorganisms. Microbial proteases are mainly produced by molds and bacteria, followed by yeast and actinomycetes.
Enzymes that catalyze the hydrolysis of proteins. There are many kinds, the important ones are pepsin, trypsin, cathepsin, papain and subtilisin. Proteases have strict selectivity for the reaction substrates they act on. A protease can only act on certain peptide bonds in protein molecules, such as the peptide bonds formed by the hydrolysis of basic amino acids catalyzed by trypsin. Proteases are widely distributed, mainly in the digestive tract of humans and animals, and are abundant in plants and microorganisms. Due to limited animal and plant resources, the industrial production of protease preparations is mainly prepared by fermentation of microorganisms such as Bacillus subtilis and Aspergillus terrestris.
Targets for Proteases
- Caspase(114)
- Aminopeptidase(24)
- ACE(74)
- Calpains(20)
- Carboxypeptidase(10)
- Cathepsin(81)
- DPP-4(31)
- Elastase(26)
- Gamma Secretase(67)
- HCV Protease(59)
- HSP(113)
- HIV Integrase(37)
- HIV Protease(47)
- MMP(228)
- NS3/4a protease(8)
- Serine Protease(18)
- Thrombin(58)
- Urokinase(4)
- Cysteine Protease(0)
- Other Proteases(18)
- Tyrosinases(47)
- 15-PGDH(1)
- Acetyl-CoA Carboxylase(13)
- Acyltransferase(59)
- Aldehyde Dehydrogenase (ALDH)(28)
- Aminoacyl-tRNA Synthetase(9)
- ATGL(1)
- Dipeptidyl Peptidase(56)
- Drug Metabolite(457)
- E1/E2/E3 Enzyme(90)
- Endogenous Metabolite(1636)
- FABP(30)
- Farnesyl Transferase(23)
- Glutaminase(14)
- Glutathione Peroxidase(14)
- Isocitrate Dehydrogenase (IDH)(28)
- Lactate Dehydrogenase(17)
- Lipoxygenase(234)
- Mitochondrial Metabolism(207)
- NEDD8-activating Enzyme(7)
- Neprilysin(12)
- PAI-1(13)
- Ser/Thr Protease(41)
- Tryptophan Hydroxylase(13)
- Xanthine Oxidase(18)
- MALT1(10)
- PCSK9(1)
Products for Proteases
- Cat.No. Nom du produit Informations
-
GC49118
10-hydroxy Warfarin
A metabolite of (R)-warfarin
-
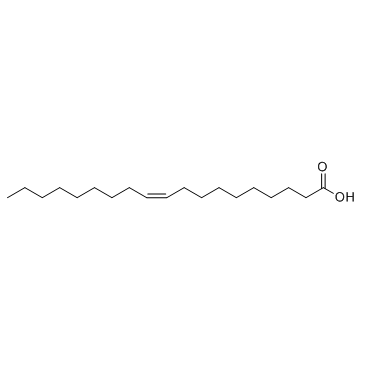
GC33800
10Z-Nonadecenoic acid
L'acide 10Z-nonadécénoÏque est une sorte d'acide gras À longue chaÎne ayant une activité anti-tumorale.
-
GC40368
11(R)-HEPE
11(R)-HEPE is produced by the oxidation of EPA by 11(R)-LO.
-
GC40445
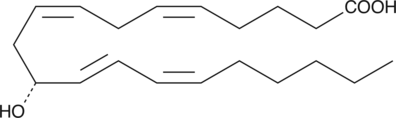
11(R)-HETE
11(R)-HETE is biosynthesized by 11(R)-LOs of the sea urchin, S.
-
GC39223
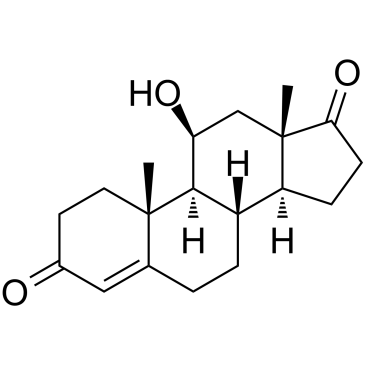
11-Beta-hydroxyandrostenedione
La 11-bêta-hydroxyandrostènedione (4-androsten-11β-ol-3,17-dione) est un stéroÏde principalement présent dans l'origine surrénalienne (la 11β-hydroxylase est présente dans le tissu surrénal, mais absente dans le tissu ovarien).
-
GC40730
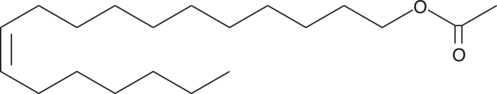
11-cis Vaccenyl Acetate
L'acétate de vaccényle 11-cis est un lipide spécifique aux mâles qui médie le comportement d'agrégation chez les mouches mâles et femelles, ce qui active quelques dizaines de neurones olfactifs situés dans la sensille T1 sur l'antenne des mouches mâles et femelles.
-
GC40394
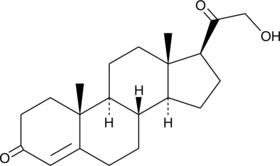
11-deoxy Corticosterone
11-désoxy Corticostérone est une hormone stéroïde produite par la glande surrénale qui possède une activité minéralocorticoïde et agit comme précurseur de l'aldostérone.
-
GC10821
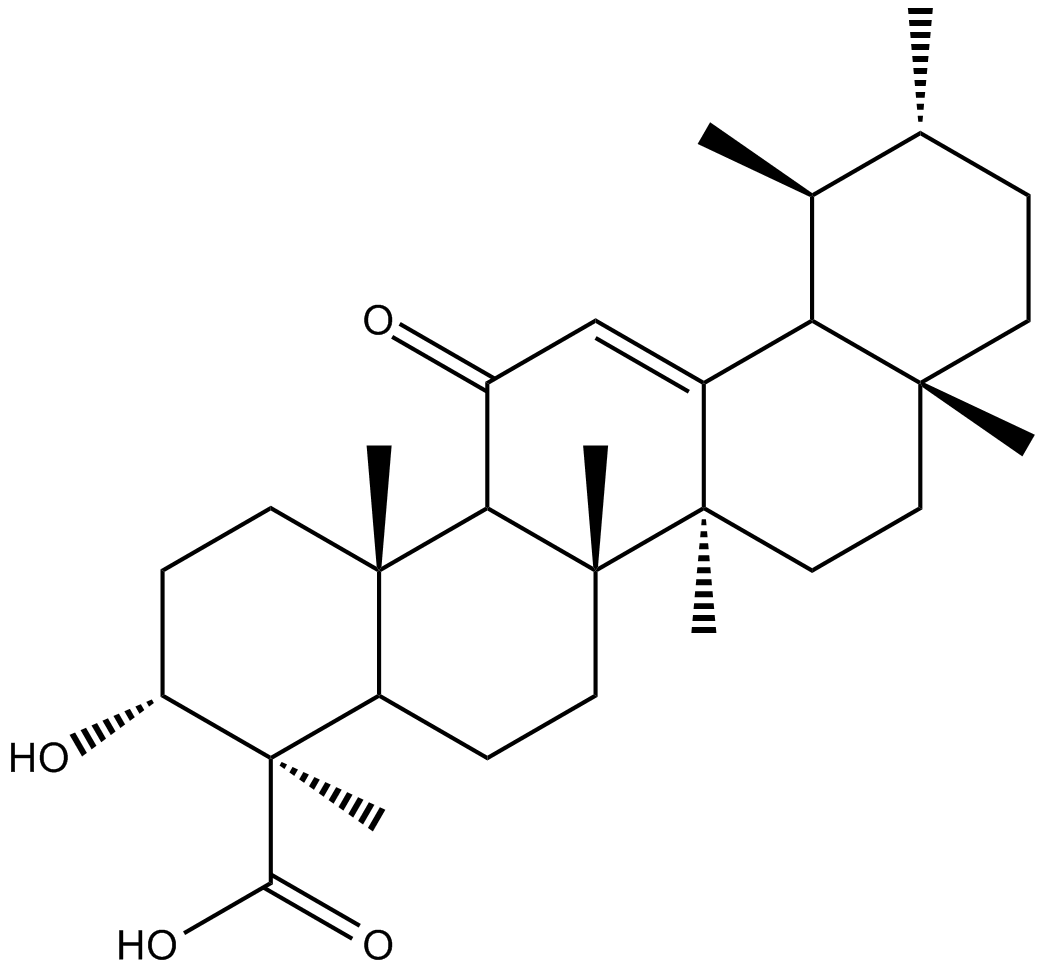
11-keto-β-Boswellic Acid
L'acide 11-céto-bêta-boswellique (11-céto-β -acide boswellique) est un acide triterpénique pentacyclique de la résine d'oléogume de l'écorce de l'arbre dentelé Boswellia, populairement connu sous le nom d'encens indien. L'acide 11-céto-bêta-boswellique a une activité anti-inflammatoire principalement due À l'inhibition de la 5-lipoxygénase (5-LOX) et de l'activation ultérieure des leucotriènes et du facteur nucléaire kappa B (NF-κ B) et de la génération alpha du facteur de nécrose tumorale production.
-
GC61538
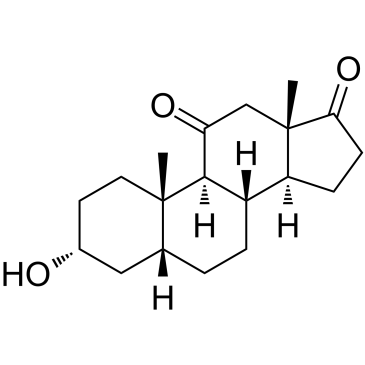
11-Oxo etiocholanolone
11-Oxo étiocholanolone (11-Ketoetiocholanolone) est un métabolite de l'étiocholanolone.
-
GC41144
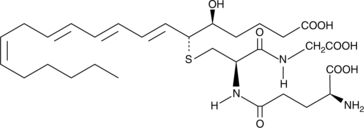
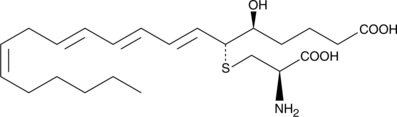
11-trans Leukotriene C4
11-trans Leukotriene C4 (11-trans LTC4) is a C-11 double bond isomer of LTC4.
-
GC41147
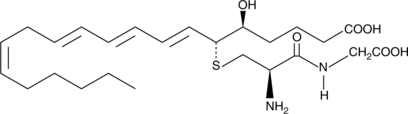
11-trans Leukotriene D4
11-trans Leukotriene D4 (11-trans LTD4) is a C-11 double bond isomer of LTD4.
-
GC41149
11-trans Leukotriene E4
Le 11-trans leucotriène E4 est un isomère du leucotriène E4 (LTE4).
-
GC63796
116-9e
116-9e (MAL2-11B) est un inhibiteur de l'ADNJA1 co-chaperon Hsp70.
-
GC34016
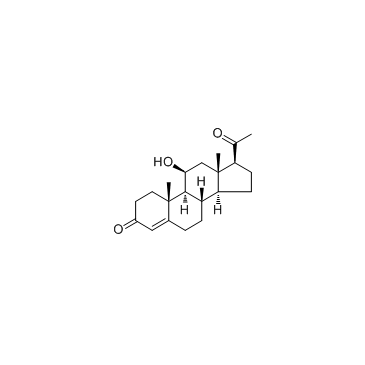
11beta-Hydroxyprogesterone (11β-Hydroxyprogesterone)
La 11bêta-hydroxyprogestérone (11&bêta ;-hydroxyprogestérone) est un puissant inhibiteur de la 11β ;-hydroxystéroÏde déshydrogénase ; active également le récepteur minéralocorticoÏde humain dans les cellules COS-7 avec une DE50 de 10 nM.
-
GC40447
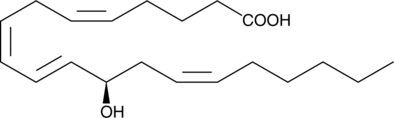
12(R)-HETE
Biosynthesis of 12(R)-HETE in invertebrates is via lipoxygenation of arachidonic acid.
-
GC40371
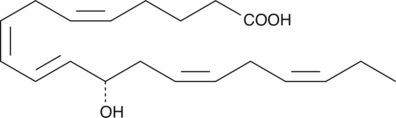
12(S)-HEPE
12(S)-HEPE is a monohydroxy fatty acid synthesized from EPA by the action of 12-LO.
-
GC40448
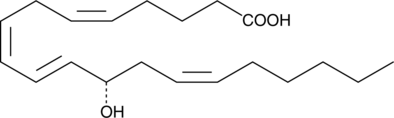
12(S)-HETE
12(S)-HETE is the predominant lipoxygenase product of mammalian platelets.
-
GC41882
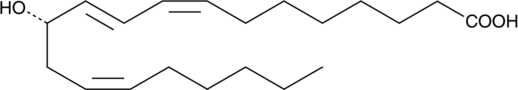
12(S)-HETrE
12(S)-HETrE is produced by 12-lipoxygenase oxidation of dihomo-γ-linolenic acid (DGLA).
-
GC41095
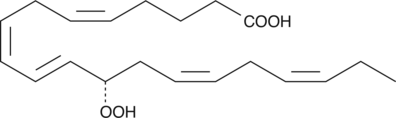
12(S)-HpEPE
12(S)-HpEPE is a monohydroperoxy polyunsaturated fatty acid produced by the action of 12-lipoxygenase on eicosapentaenoic acid.
-
GC41122
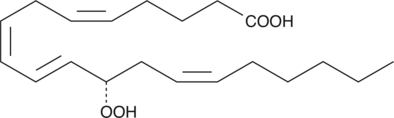
12(S)-HpETE
12(S)-HpETE is a monohydroperoxy polyunsaturated fatty acid (PUFA) produced by the action of platelet or leukocyte 12-lipoxygenase (12-LO) on arachidonic acid.
-
GC41123
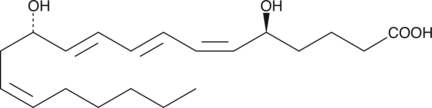
12-epi Leukotriene B4
Leukotriene B4 (LTB4) compounds are produced by both enzymatic and non-enzymatic processes.
-
GC45962
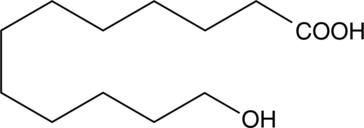
12-hydroxy Lauric Acid
L'acide 12-hydroxylaurique est un métabolite endogène.
-
GC60443
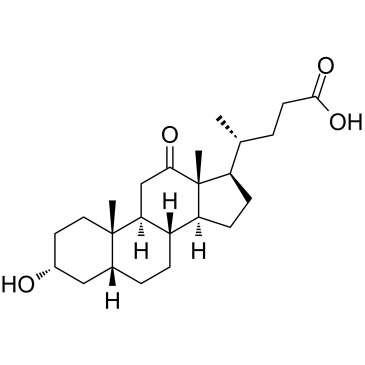
12-Ketodeoxycholic acid
L'acide 12-cétodésoxycholique est un acide biliaire, métabolite du rein.
-
GC41096
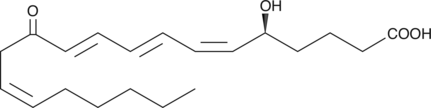
12-oxo Leukotriene B4
Leukotriene B4 (LTB4) is a dihydroxy fatty acid derived from arachidonic acid through the 5-LO pathway.
-
GC46418
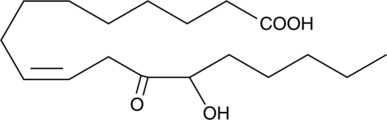
12-oxo-13-HOME
An oxylipin
-
GC40372
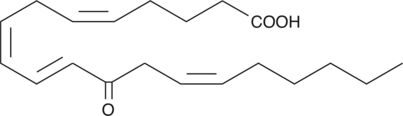
12-OxoETE
12-OxoETE is synthesized by human platelets and Aplysia nervous tissue after incubation with arachidonic acid.
-
GC19462
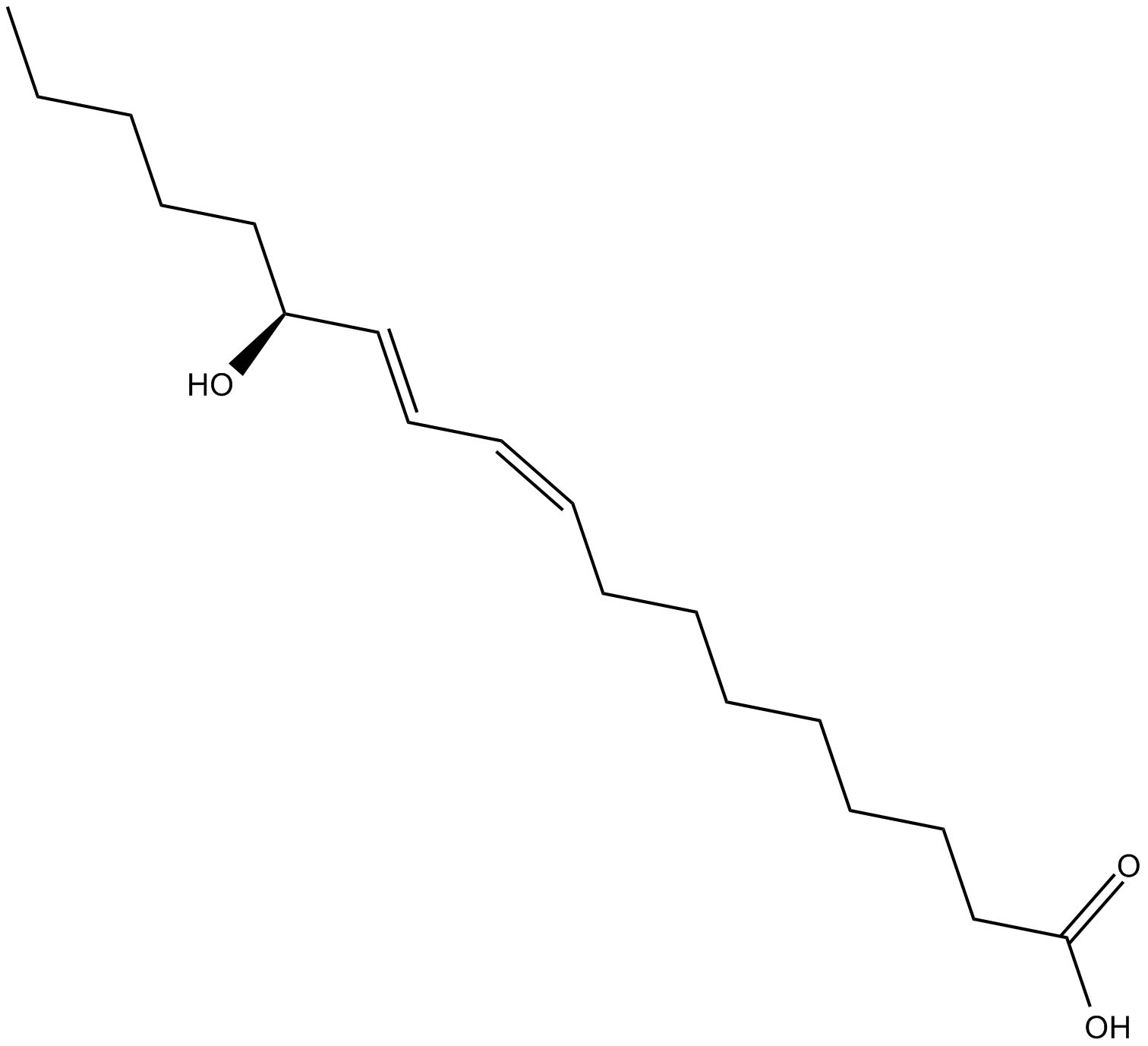
13(R)-HODE
13(R)-HODE is the opposite enantiomer of the 13(S)-HODE produced when linoleic acid is incubated with soybean lipoxygenase.

-
GC19463
13(S)-HODE
13(S)-HODE (13(S)-HODE), le produit du métabolisme de la 15-lipoxygénase (15-LOX) de l'acide linoléique, fonctionne comme le ligand endogène pour activer PPARγ.
-
GC41220
13(S)-HODE methyl ester
13(S)-hydroxyoctadecadienoic acid (13(S)-HODE) is a 15-lipoxygenase metabolite of linoleic acid produced in endothelial cells, leukocytes, and tumor cells.
-
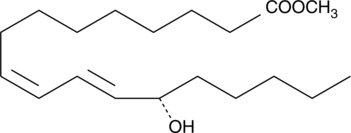
GC41896
13(S)-HODE-biotin
13(S)-HODE is the lipoxygenase metabolite of linoleic acid.
-
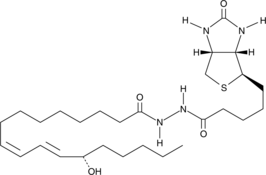
GC46420
13(S)-HODE-d4
An internal standard for the quantification of 13-HODE
-
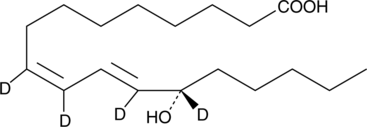
GC41897
13(S)-HOTrE
13(S)-HOTrE is the 15-lipoxygenase (15-LO) product of linolenic acid.
-
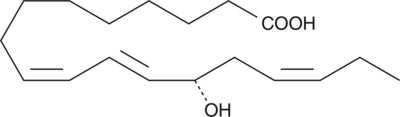
GC41898
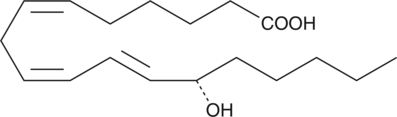
13(S)-HOTrE(γ)
13(S)-HOTrE(γ) is the 15-LO product of γ-linolenic acid.
-
GC19474
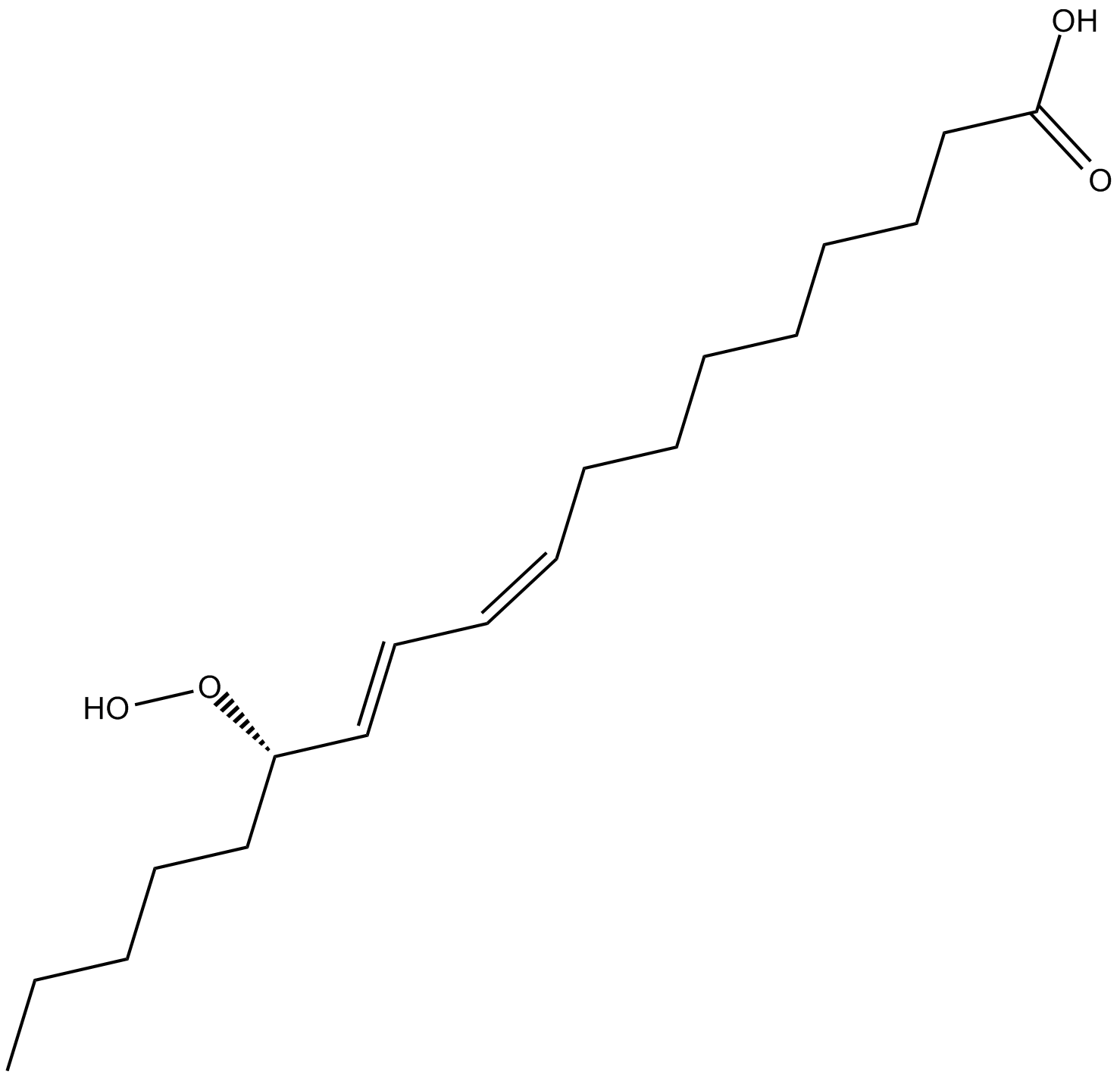
13(S)-HpODE
13(S)-HpODE is produced by the oxidation of linoleic acid by lipoxygenase-1 (LO-1) in many plants including soybean, flaxseed, apples, and tea leaves,1,2 and by 15-LO in mammals.
-
GC41899
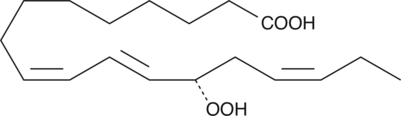
13(S)-HpOTrE
13(S)-HpOTrE is a monohydroperoxy polyunsaturated fatty acid produced in soybeans by the action of soybean LO-2 on esterified α-linolenic acid.
-
GC41900
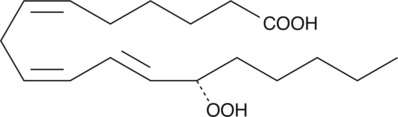
13(S)-HpOTrE(γ)
13(S)-HpOTrE(γ) is a monohydroxy PUFA produced by the action of soybean lipoxygenase-1 (LO-1) on γ-linolenic acid.
-
GC62758
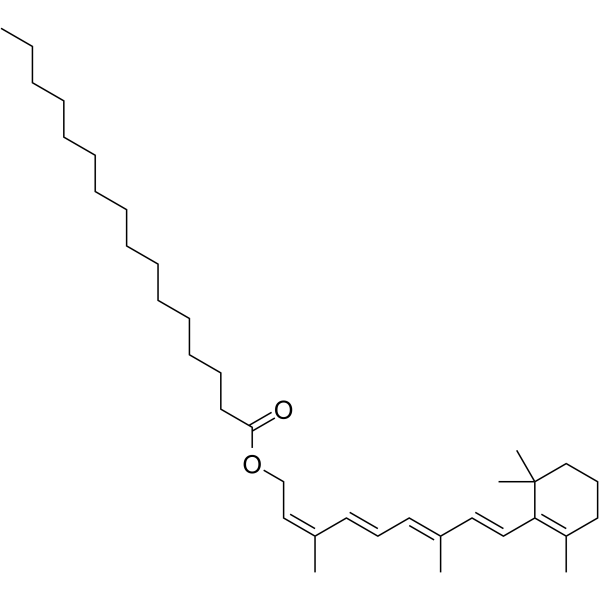
13-cis-Vitamin A palmitate
Le palmitate de 13-cis-vitamine A (palmitate de 13-cis-rétinyle) est un isomère 13-cis formé par le palmitate de vitamine A dans les flocons de maÏs.
-
GC41911
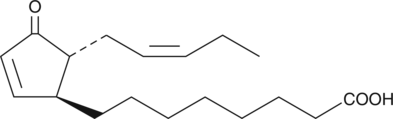
13-epi-12-oxo Phytodienoic Acid
13-epi-12-oxo Phytodienoic acid (13-epi-12-oxo PDA) is a lipoxygenase metabolite of α-linolenic acid in the leaves of green plants such as corn.
-
GC49759
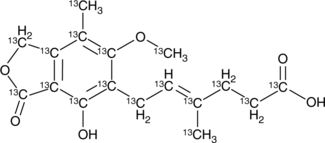
13C17-Mycophenolic Acid
An internal standard for the quantification of mycophenolic acid
-
GC41206
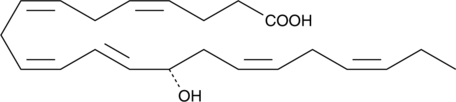
14(S)-HDHA
La 14(S)-HDHA (14(S)-HDoHE) est un produit d'oxygénation de l'acide docosahexaénoÏque (DHA).
-
GC41100
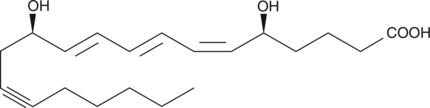
14,15-dehydro Leukotriene B4
Leukotriene B4 (LTB4) is a dihydroxy fatty acid derived from arachidonic acid through the 5-lipoxygenase pathway.
-
GC41145
14,15-Leukotriene C4
Leukotrienes (LTs) are a group of acute inflammatory mediators derived from arachidonic acid in leukocytes.
-
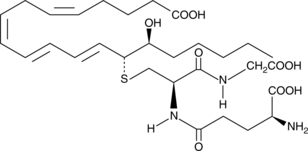
GC41148
14,15-Leukotriene D4
14,15-Leukotriene D4 (14,15-LTD4) is a member of an alternate class of LTs synthesized by a pathway involving the dual actions of 15- and 12-lipoxygenases (15- and 12-LOs) on arachidonic acid via 15-HpETE and 14,15-LTA4 intermediates.
-
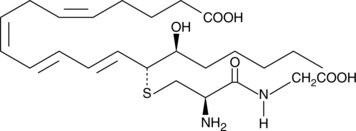
GC41150
14,15-Leukotriene E4
Leukotrienes (LTs) are a group of acute inflammatory mediators derived from arachidonic acid in leukocytes.
-
GC41415
15(R)-Lipoxin A4
Lipid-derived lipoxins are produced at the site of vascular and mucosal inflammation where they down-regulate polymorphonuclear leukocyte recruitment and function.
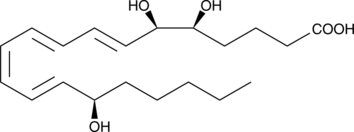
-
GC40427
15(S)-HEDE
15(S)-HEDE is produced from 11Z,14Z-eicosadienoic acid by 15-LO.
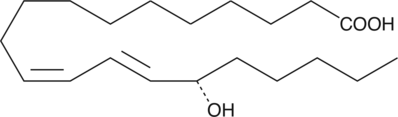
-
GC40373
15(S)-HEPE
15(S)-HEPE is a monohydroxy fatty acid synthesized from EPA by the action of 15-LO.
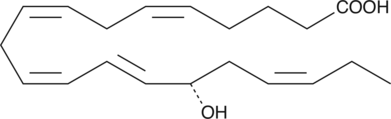
-
GC40451
15(S)-HETE
15(S)-HETE is a major arachidonic acid metabolite from the 15-lipoxygenase pathway.
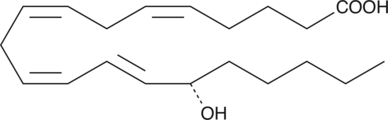
-
GC41925
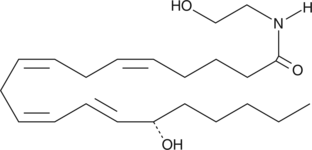
15(S)-HETE Ethanolamide
Arachidonoyl ethanolamide was the first endogenous cannabinoid (CB) to be isolated and characterized as an agonist acting on the same receptors (CB1 and CB2) as THC.
-
GC40839
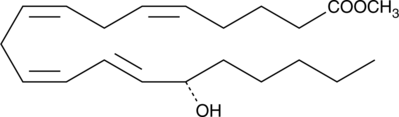
15(S)-HETE methyl ester
15(S)-HETE methyl ester is a synthetic derivative of 15(S)-HETE, a major arachidonic acid metabolite from the 15-lipoxygenase pathway.
-
GC46442
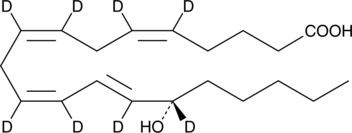
15(S)-HETE-d8
An internal standard for the quantification of 15-HETE
-
GC49894
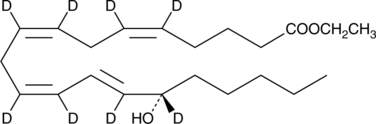
15(S)-HETE-d8 ethyl ester
An internal standard for the quantification of 15(S)-HETE ethyl ester
-
GC41927
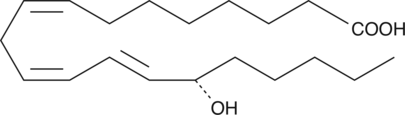
15(S)-HETrE
15(S)-HETrE is the hydroxy-trienoic acid resulting from 15-lipoxygenation of dihomo-γ-linolenic acid.
-
GC41403
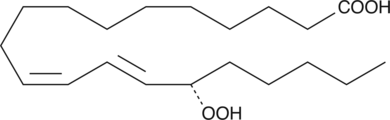
15(S)-HpEDE
15(S)-HpEDE is a monohydroperoxy polyunsaturated fatty acid produced by the action of 15-lipoxygenase on eicosadienoic acid.
-
GC41101
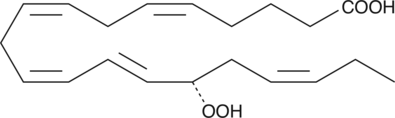
15(S)-HpEPE
15(S)-HpEPE is a monohydroperoxy polyunsaturated fatty acid produced by the action of 15-lipoxygenase on eicosapentaenoic acid.
-
GC41124
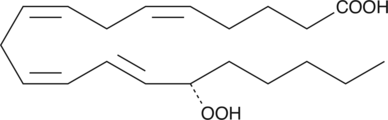
15(S)-HpETE
15(S)-HpETE is a monohydroperoxy polyunsaturated fatty acid (PUFA) produced by the action of 15-lipoxygenase (15-LO) on arachidonic acid.
-
GC11988
15-acetoxy Scirpenol
Le 15-acétoxyscirpénol, l'une des mycotoxines du groupement acétoxyscirpénol (ASM), induit fortement l'apoptose et inhibe la croissance des lymphocytes T Jurkat de manière dose-dépendante en activant d'autres caspases indépendantes de la caspase-3.
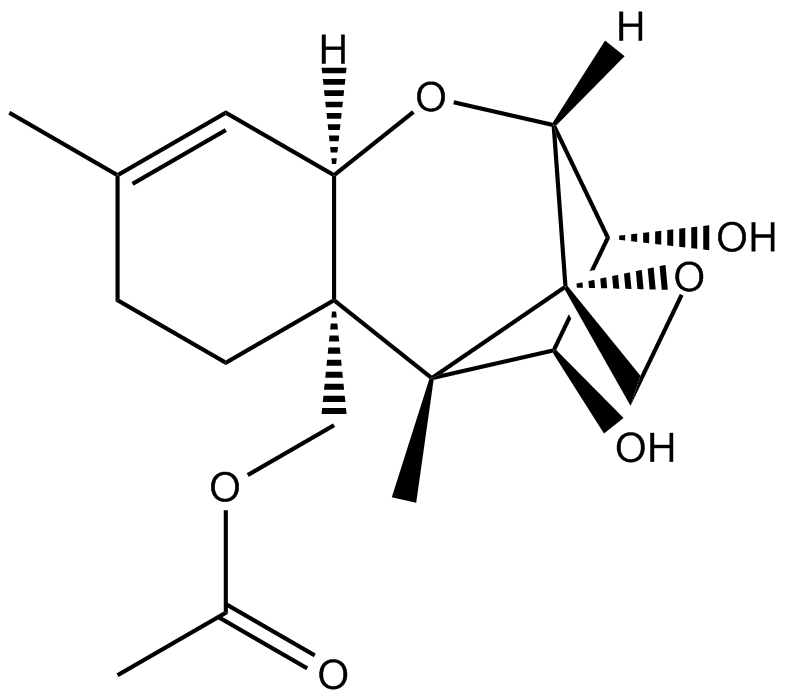
-
GC19442
15-Acetyldeoxy Nivalenol
Le 15-acétyldésoxy nivalénol est un trichothécène hautement toxique présent dans les céréales, et un métabolite du désoxynivalénol, présente une toxicité pour les cellules HepG2.

-
GC41937
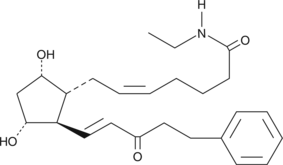
15-keto-17-phenyl trinor Prostaglandin F2α ethyl amide
Bimatoprost is the Allergan trade name for 17-phenyl trinor prostaglandin F2α ethyl amide (17-phenyl trinor PGF2α ethyl amide), an F-series PG analog which has been approved for use as an ocular hypotensive drug.
-
GC41938
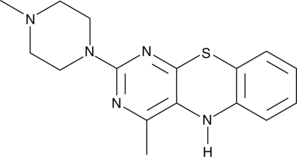
15-Lipoxygenase Inhibitor 1
L'inhibiteur de 15-lipoxygénase 1 est un inhibiteur sélectif de la 15-lipoxygénase, avec une CI50 de 18 μM. L'inhibiteur de la 15-lipoxygénase 1 a des CI50 de 19,5 μM et 19,1 μM pour la 15-lipoxygénase de soja (SLO) et la 15-lipoxygénase-1 humaine (15-LOX-1), respectivement. L'inhibiteur de la 15-lipoxygénase 1 a un potentiel pour la recherche sur le cancer de la prostate.
-
GC41940
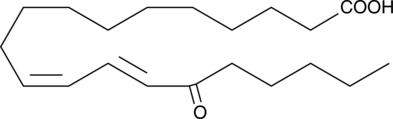
15-OxoEDE
15-OxoEDE is produced by the oxidation of 15-HEDE.
-
GC40376
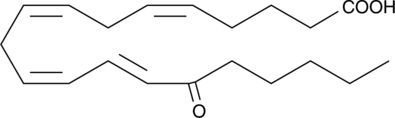
15-OxoETE
15-OxoETE is produced by oxidation of the 15-hydroxyl of 15-HETE.
-
GC41309
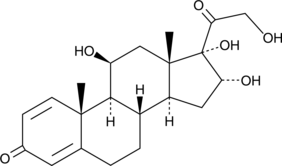
16α-hydroxy Prednisolone
16α-hydroxy Prednisolone est un métabolite stéréosélectif de l'épimère 22(R) du budésonide glucocorticoÏde via les enzymes du cytochrome P450 3A (CYP3A).
-
GC35058
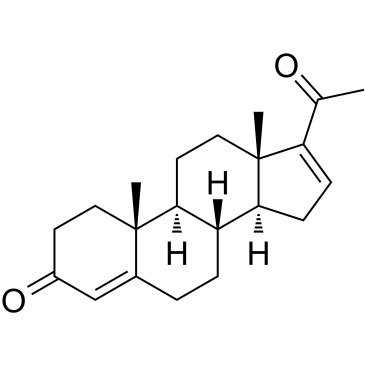
16-Dehydroprogesterone
La 16-déhydroprogestérone est un progestatif stéroïdien.
-
GC46451
16F16
A PDI inhibitor

-
GC45909
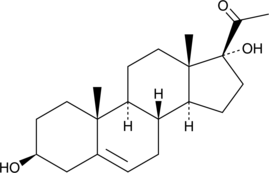
17α-hydroxy Pregnenolone
1α-hydroxy Pregnenolone est un stéroÏde pregnane.
-
GC41300
17β-hydroxy Exemestane
17β-hydroxy Exemestane is the primary active metabolite of exemestane.

-
GC41951
17(R)-Resolvin D1
Resolvins are a family of potent lipid mediators derived from both eicosapentaenoic acid and docosahexaenoic acid.
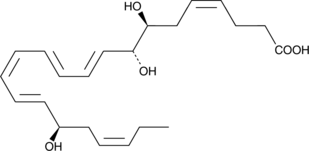
-
GC41227
17(R)-Resolvin D1 methyl ester
17(R)-Resolvin D1 (17(R)-RvD1) is an aspirin-triggered epimer of RvD1 that reduces human polymorphonuclear leukocyte transendothelial migration, the earliest event in acute inflammation, with equipotency to RvD1 (EC50 = ~30 nM).
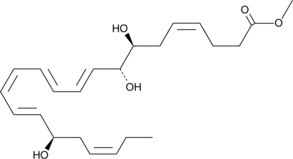
-
GC41952
17(R)-Resolvin D4
17(R)-Resolvin D4 (17(R)-RvD4) is an aspirin-triggered epimer of RvD4 .
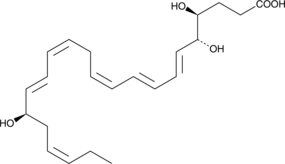
-
GC41208
17(S)-HDHA
17(S)-HDHA is a primary mono-oxygenation product of docosahexaenoic acid in human whole blood, human leukocytes, and mouse brain.
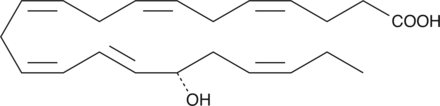
-
GC49356
17(S)-HDoTE
A metabolite of adrenic acid
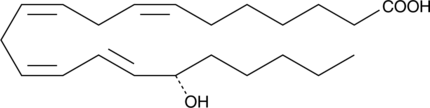
-
GC40975
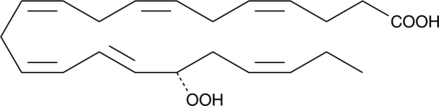
17(S)-HpDHA
17(S)-HpDHA is a mono-oxygenation product of docosahexaenoic acid in human whole blood, human leukocytes, human glial cells, and mouse brain.
-
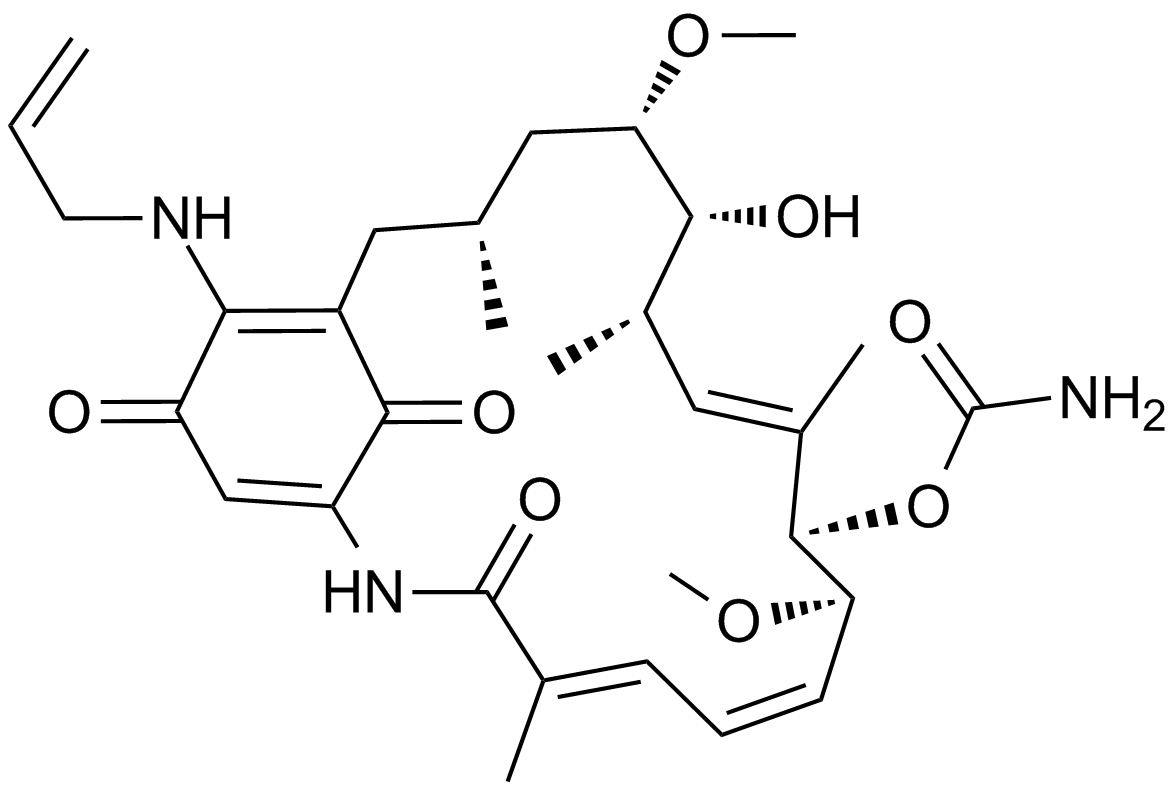
GC11720
17-AAG (KOS953)
An inhibitor of Hsp90
-
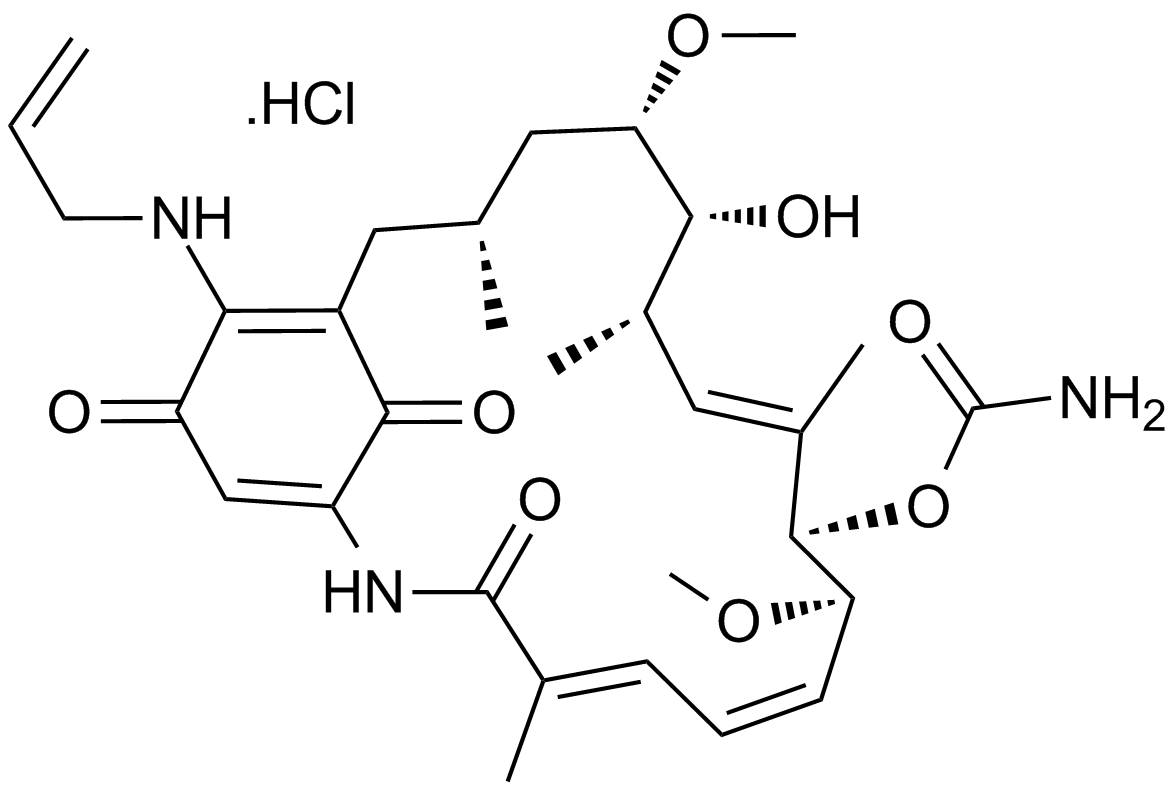
GC17210
17-AAG Hydrochloride
Hsp90 inhibitor,geldanamycin analogue
-
GC41955
17-DMAG
17-DMAG (17-DMAG) est un puissant inhibiteur de Hsp90, se liant À Hsp90 avec une CE50 de 62 ± ; 29 nM.
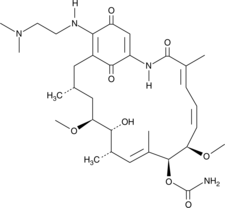
-
GC13044
17-DMAG (Alvespimycin) HCl
Le 17-DMAG (alvespimycine) HCl (chlorhydrate de 17-DMAG ; KOS-1022 ; BMS 826476) est un puissant inhibiteur de Hsp90, se liant À Hsp90 avec une EC50 de 62± ; 29 nM.

-
GC41529
17-oxo-4(Z),7(Z),10(Z),13(Z),15(E),19(Z)-Docosahexaenoic Acid
17-oxo-4(Z),7(Z),10(Z),13(Z),15(E),19(Z)-Docosahexaenoic acid is a metabolite of lipoxygenase-mediated oxidation of DHA that is produced endogenously by aspirin-enhanced COX-2 activity.
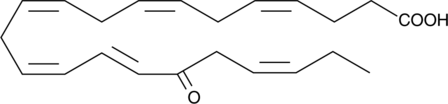
-
GC41209
17-oxo-7(Z),10(Z),13(Z),15(E),19(Z)-Docosapentaenoic Acid
Docosapentaenoic acid (DPA) is a ω-3 fatty acid found in fish oils.
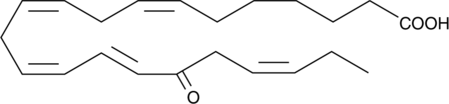
-
GC68426
17a-Hydroxypregnenolone-d3
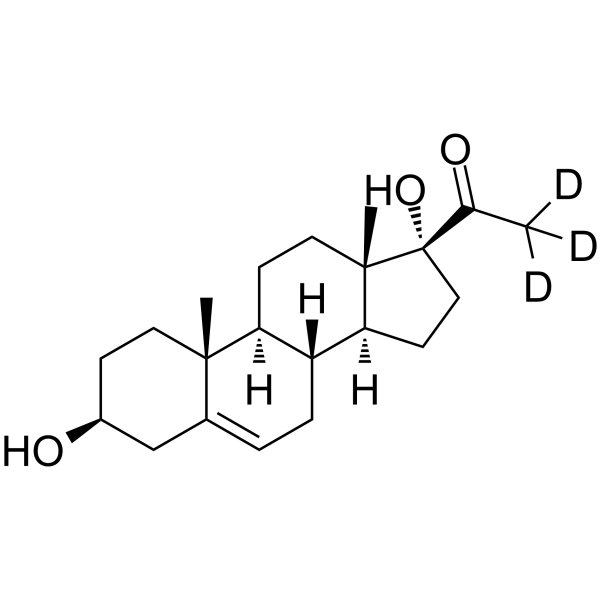
-
GC35061
18α-Glycyrrhetinic acid
18α-L'acide glycyrrhétinique, un composé dérivé de l'alimentation, est un inhibiteur de NF-kB et un activateur du protéasome, qui sert de facteur de pro-longévité et d'anti-agrégation dans un organisme multicellulaire.
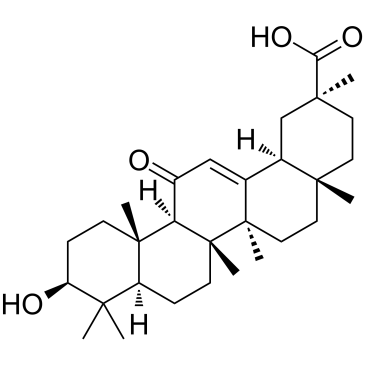
-
GC41980
18-carboxy dinor Leukotriene B4
18-carboxy dinor Leukotriene B4 (18-carboxy dinor LTB4) is a β-oxidation metabolite of LTB4.
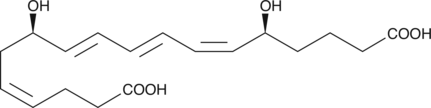
-
GC33818
18-Hydroxycorticosterone
La 18-hydroxycorticostérone est un corticostéroÏde et un dérivé de la corticostérone, qui peut entraÎner de graves déséquilibres électrolytiques.
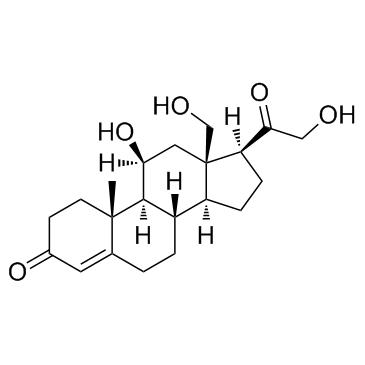
-
GC63603
19-Hydroxyandrost-4-ene-3,17-dione
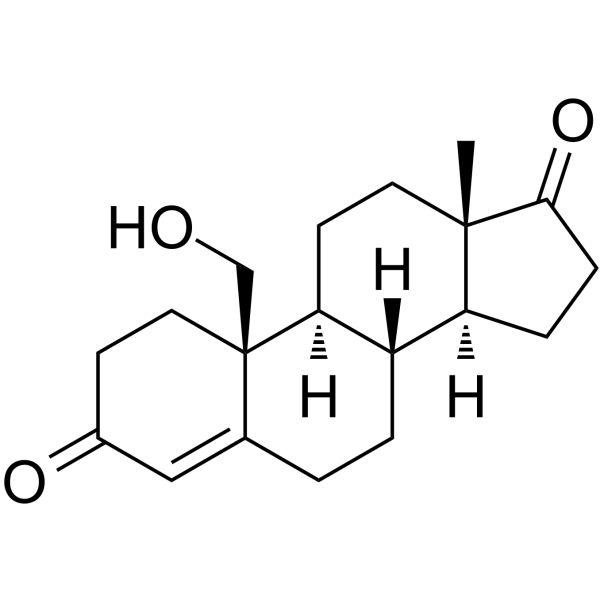
-
GC39296
1G244
1G244 est un puissant inhibiteur de DPP8/9 avec des IC50 de 12 nM et 84 nM, respectivement. 1G244 n'inhibe pas DPPIV et DPPII. 1G244 induit l'apoptose dans les cellules de myélome multiple et a des effets anti-myélome.
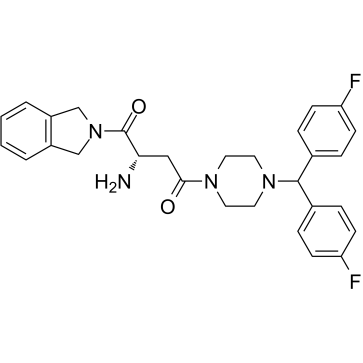
-
GC38359
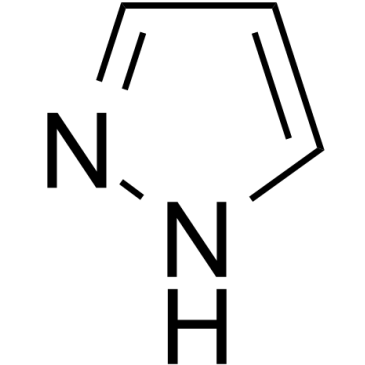
1H-pyrazole
Le 1H-pyrazole est un métabolite endogène.
-
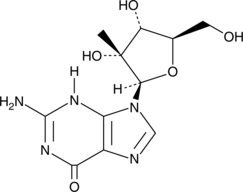
GC49823
2′-C-β-Methylguanosine
An active nucleoside metabolite of BMS-986094
-
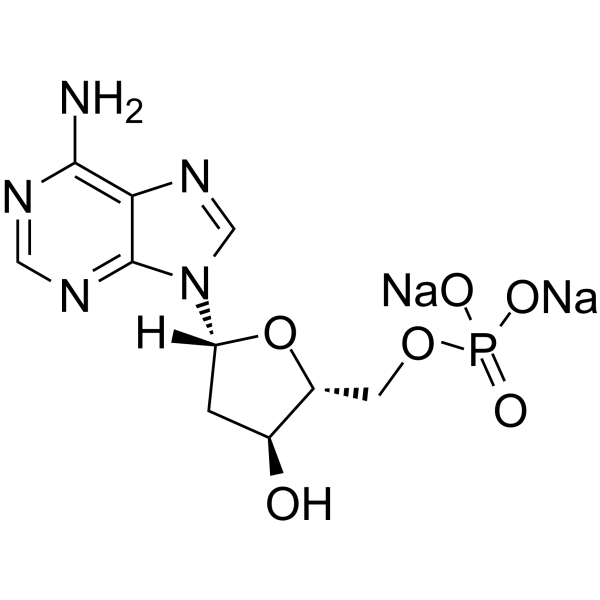
GC64738
2′-Deoxyadenosine 5′-monophosphate disodium
&2#8242;-Déoxyadénosine 5′-monophosphate disodique, un dérivé de l'acide nucléique AMP, est un désoxyribonucléotide présent dans l'ADN.
-
GC62772
2’-Deoxyadenosine-5’-triphosphate trisodium
&2rsquo;-Déoxyadénosine-5’-triphosphate trisodique (dATP trisodique) est un nucléotide utilisé dans les cellules pour la synthèse (ou la réplication) de l'ADN, en tant que substrat de l'ADN polymérase.
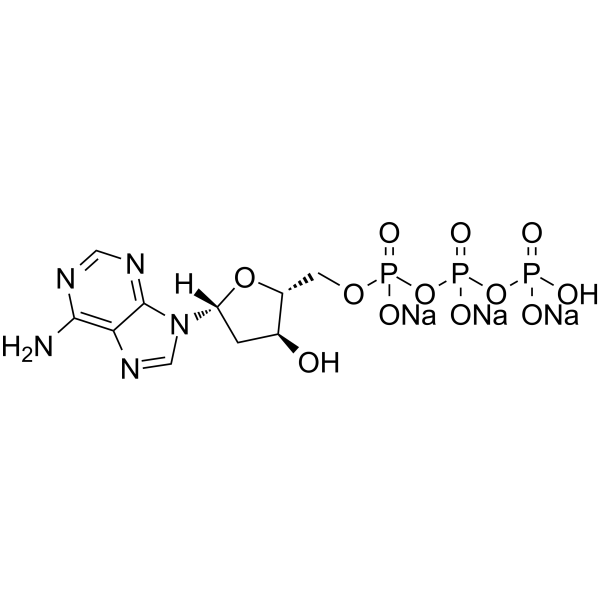
-
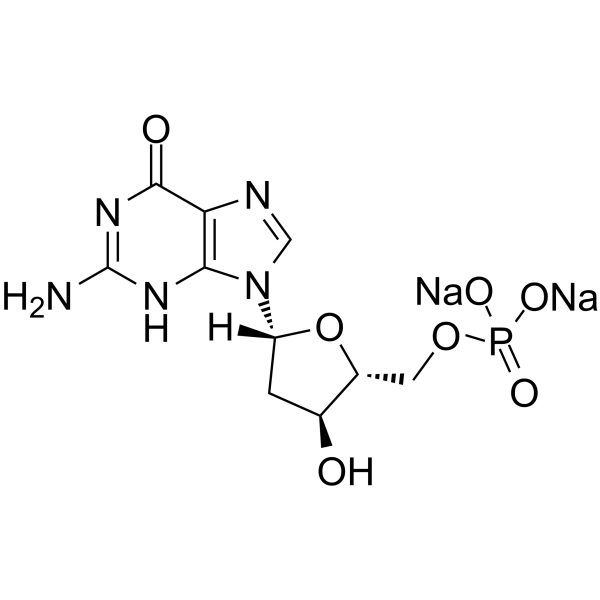
GC62774
2’-Deoxyguanosine 5’-monophosphate disodium
&2rsquo;-Deoxyguanosine 5’-monophosphate disodique (5′-dGMP disodique) est un mononucléotide ayant la guanine comme base nucléique.
-
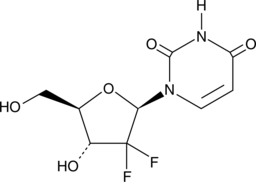
GC46508
2',2'-Difluoro-2'-deoxyuridine
An active metabolite of gemcitabine
-
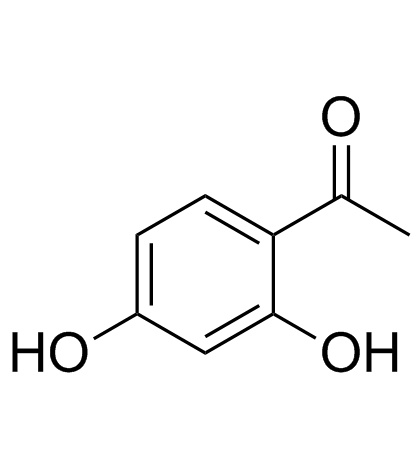
GC33622
2',4'-Dihydroxyacetophenone
La 2',4'-dihydroxyacétophénone (résacétophénone) est une acétophénone portant des substituants hydroxy aux positions 2' et 4'.
-
GC60462
2',4'-Dimethylacetophenone
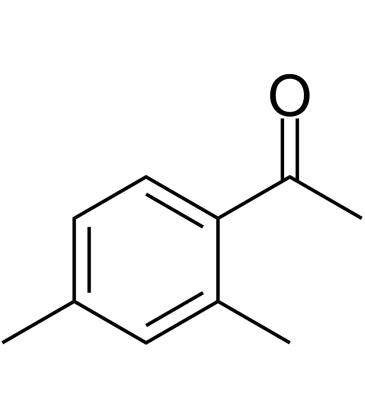
-
GC33605
2'-Deoxyadenosine monohydrate
Le monohydrate de 2'-désoxyadénosine est un désoxyribonucléoside.
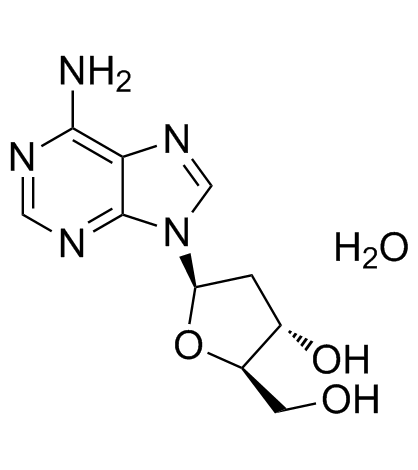
-
GC38258
2'-Deoxyadenosine-5'-monophosphate
Le 2′-désoxyadénosine 5′-monophosphate, un dérivé de l'acide nucléique AMP, est un désoxyribonucléotide présent dans l'ADN.
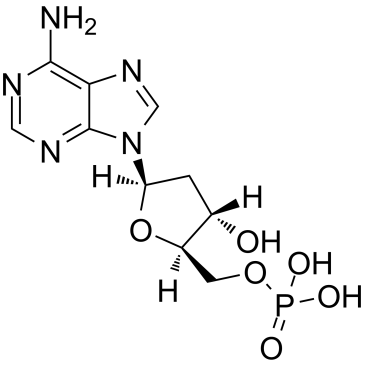
-
GC42150
2'-Deoxycytidine 5'-diphosphate (sodium salt hydrate)
2'-Deoxycytidine-5'-diphosphate (dCDP) trisodique est un métabolite endogène.
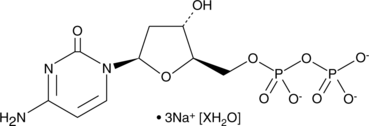
-
GC17436
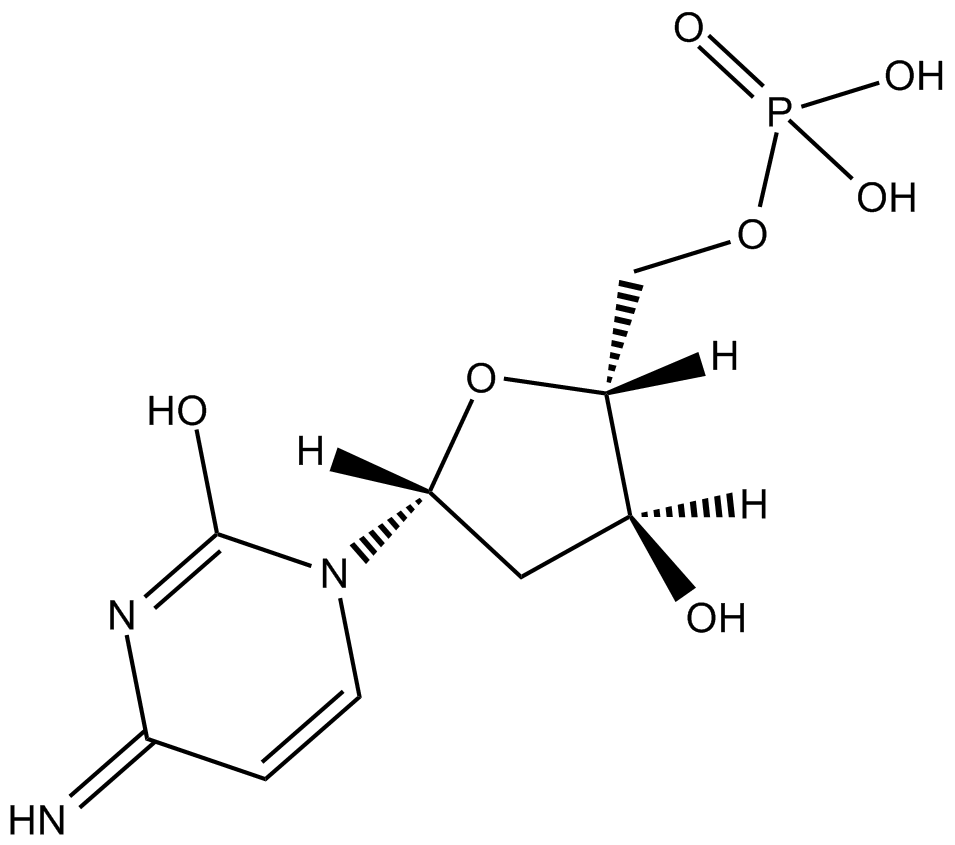
2'-Deoxycytidine-5'-monophosphoric acid
L'acide 2'-désoxycytidine-5'-monophosphorique est un métabolite endogène.
-
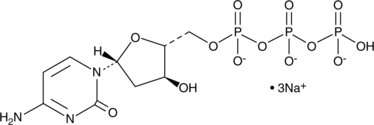
GC48440
2'-Deoxycytidine-5'-triphosphate (sodium salt)
2'-Deoxycytidine-5'-triphosphate (sel de sodium) (dCTP sel trisodique) est un nucléoside triphosphate qui peut être utilisé pour la synthèse d'ADN.
-
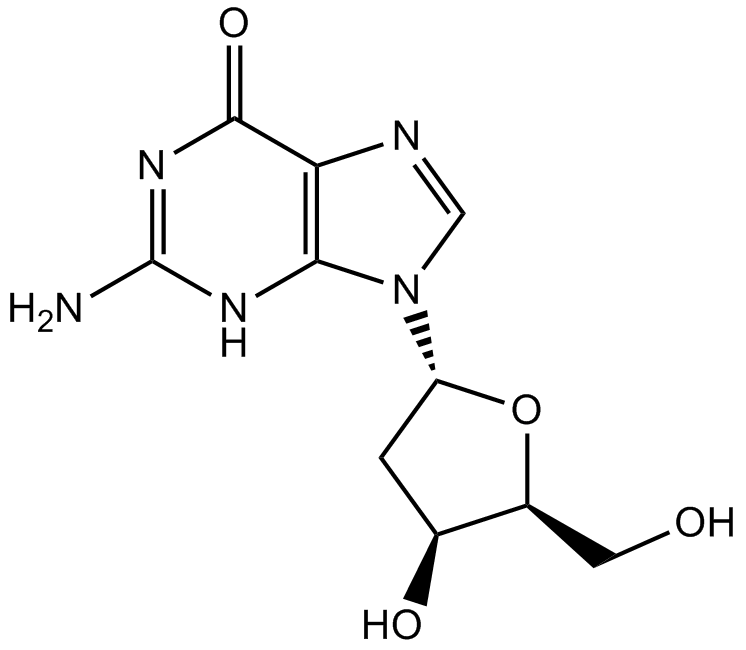
GC10897
2'-Deoxyguanosine
La 2'-désoxyguanosine (désoxyguanosine) est composée de la guanine nucléoside purique liée par son azote N9 au carbone C1 du désoxyribose.
-
GC38191
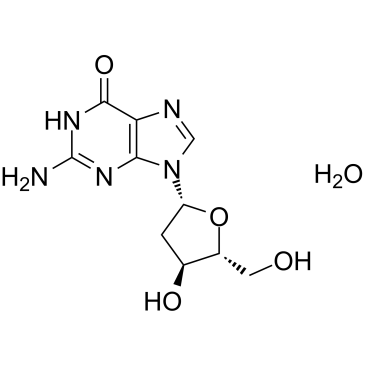
2'-Deoxyguanosine monohydrate
Le monohydrate de 2'-désoxyguanosine est un métabolite endogène.