Proteases
Proteases is a general term for a class of enzymes that hydrolyze protein peptide chains. According to the way they degrade polypeptides, they are divided into two categories: endopeptidases and telopeptidases. The former can cut the large molecular weight polypeptide chain from the middle to form prions and peptones with smaller molecular weights; the latter can be divided into carboxypeptidase and aminopeptidase, which respectively remove the peptide from the free carboxyl terminus or free amino terminus of the polypeptide one by one. Chain hydrolysis produces amino acids.
A general term for a class of enzymes that hydrolyze peptide bonds in proteins. According to the way they hydrolyze polypeptides, they can be divided into endopeptidases and exopeptidases. Endopeptidase cleaves the interior of the protein molecule to form smaller molecular weight peptones and peptones. Exopeptidase hydrolyzes peptide bonds one by one from the end of the free amino group or carboxyl group of protein molecules, and frees amino acids, the former is aminopeptidase and the latter is carboxypeptidase. Proteases can be classified into serine proteases, sulfhydryl proteases, metalloproteases and aspartic proteases according to their active centers and optimum pH. According to the optimum pH value of its reaction, it is divided into acidic protease, neutral protease and alkaline protease. The proteases used in industrial production are mainly endopeptidases.
Proteases are widely found in animal offal, plant stems and leaves, fruits and microorganisms. Microbial proteases are mainly produced by molds and bacteria, followed by yeast and actinomycetes.
Enzymes that catalyze the hydrolysis of proteins. There are many kinds, the important ones are pepsin, trypsin, cathepsin, papain and subtilisin. Proteases have strict selectivity for the reaction substrates they act on. A protease can only act on certain peptide bonds in protein molecules, such as the peptide bonds formed by the hydrolysis of basic amino acids catalyzed by trypsin. Proteases are widely distributed, mainly in the digestive tract of humans and animals, and are abundant in plants and microorganisms. Due to limited animal and plant resources, the industrial production of protease preparations is mainly prepared by fermentation of microorganisms such as Bacillus subtilis and Aspergillus terrestris.
Targets for Proteases
- Caspase(114)
- Aminopeptidase(24)
- ACE(74)
- Calpains(20)
- Carboxypeptidase(10)
- Cathepsin(81)
- DPP-4(31)
- Elastase(26)
- Gamma Secretase(67)
- HCV Protease(59)
- HSP(113)
- HIV Integrase(37)
- HIV Protease(47)
- MMP(228)
- NS3/4a protease(8)
- Serine Protease(18)
- Thrombin(58)
- Urokinase(4)
- Cysteine Protease(0)
- Other Proteases(18)
- Tyrosinases(47)
- 15-PGDH(1)
- Acetyl-CoA Carboxylase(13)
- Acyltransferase(59)
- Aldehyde Dehydrogenase (ALDH)(28)
- Aminoacyl-tRNA Synthetase(9)
- ATGL(1)
- Dipeptidyl Peptidase(56)
- Drug Metabolite(457)
- E1/E2/E3 Enzyme(90)
- Endogenous Metabolite(1636)
- FABP(30)
- Farnesyl Transferase(23)
- Glutaminase(14)
- Glutathione Peroxidase(14)
- Isocitrate Dehydrogenase (IDH)(28)
- Lactate Dehydrogenase(17)
- Lipoxygenase(234)
- Mitochondrial Metabolism(207)
- NEDD8-activating Enzyme(7)
- Neprilysin(12)
- PAI-1(13)
- Ser/Thr Protease(41)
- Tryptophan Hydroxylase(13)
- Xanthine Oxidase(18)
- MALT1(10)
- PCSK9(1)
Products for Proteases
- Cat.No. Product Name Information
-
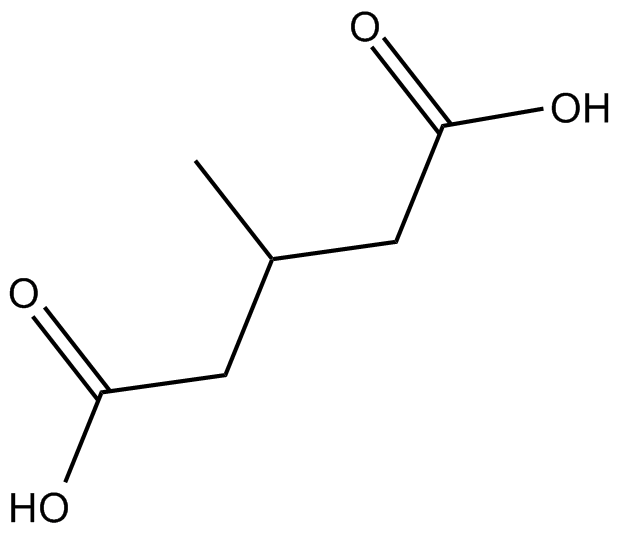
GC16812
3-Methylglutaric acid
A metabolite of L-leucine
-
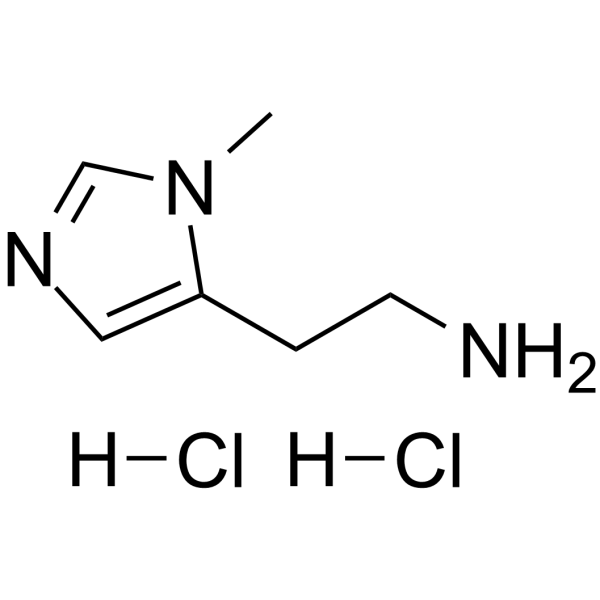
GC62795
3-Methylhistamine dihydrochloride
3-Methylhistamine dihydrochloride is a degradation product of histamine.
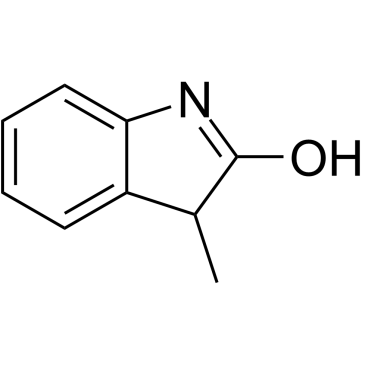
-
GC38297
3-Methylindolin-2-one
-
GC33525
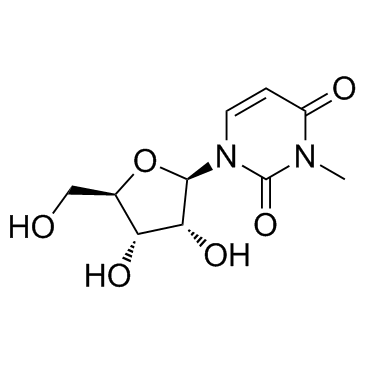
3-Methyluridine
3-Methyluridine (N3-Methyluridine) is a modified RNA nucleoside.
-
GC62796
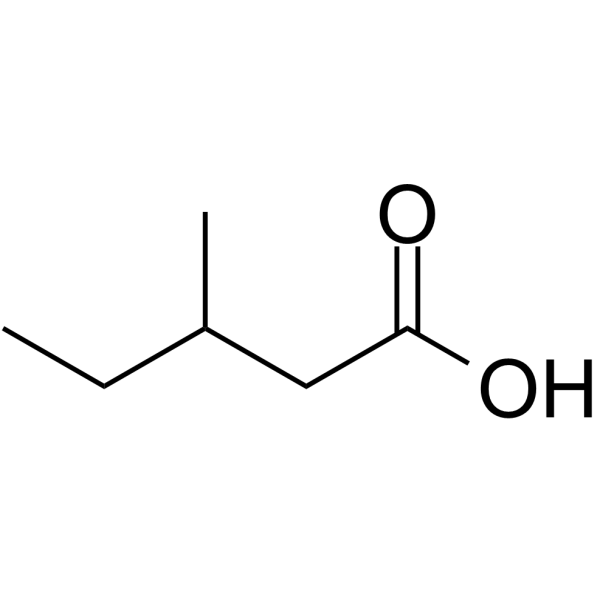
3-Methylvaleric Acid
3-Methylvaleric Acid is a flavouring ingredient.
-
GC30635
3-Methylxanthine
A cGMP PDE inhibitor and metabolite of theophylline and caffeine

-
GC64078
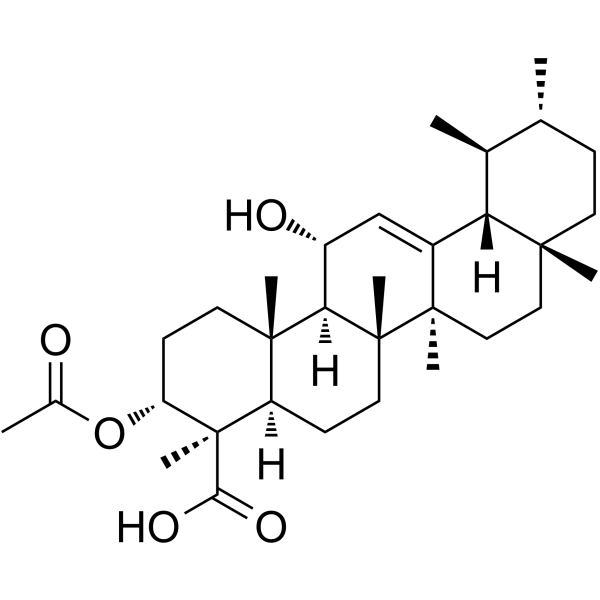
3-O-Acetyl-11-hydroxy-beta-boswellic acid
3-O-Acetyl-11-hydroxy-beta-boswellic acid is a potent 5-lipoxygenase (5-LO) inhibitor.
-
GC61717
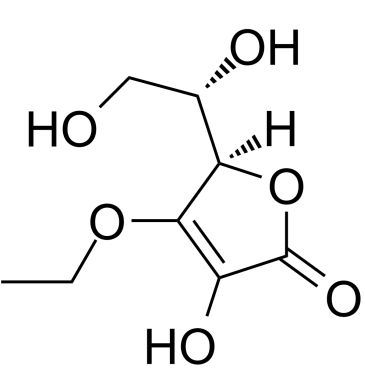
3-O-Ethyl-L-ascorbic acid
A tyrosinase inhibitor
-
GC42307
3-O-methyl-L-DOPA (hydrate)
3-O-methyl-L-DOPA is a metabolite of L-DOPA, produced by the activity of catechol O-methyltransferase.
-
GC60505
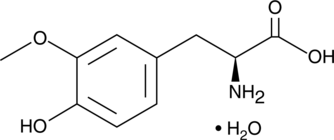
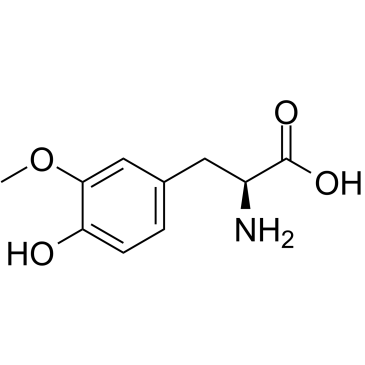
3-O-Methyldopa
3-O-Methyldopa (3-Methoxy-L-tyrosine) is a metabolite of L-DOPA which is formed by catechol-O-methyltransferase (COMT).
-
GC60506
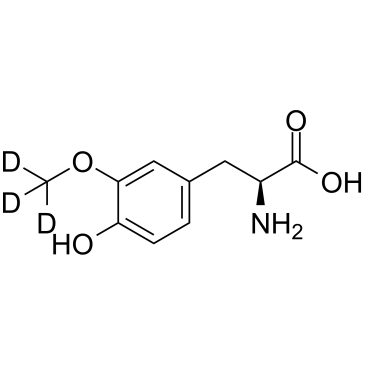
3-O-Methyldopa D3
3-O-Methyldopa D3 (3-Methoxy-L-tyrosine-d3) is deuterium labeled 3-O-Methyldopa.
-
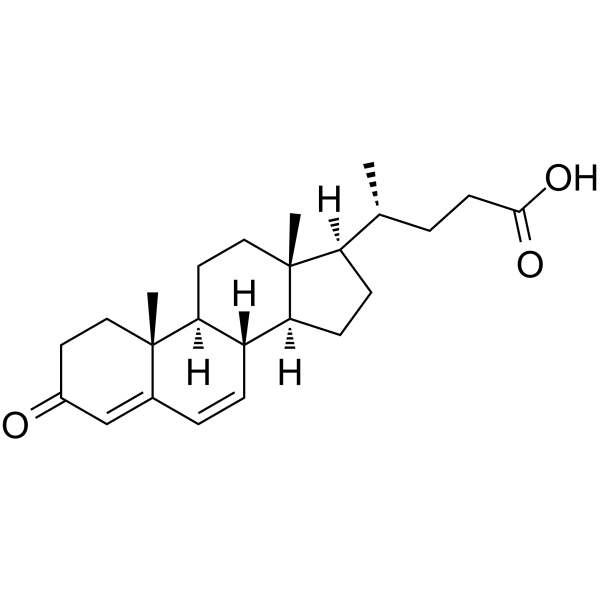
GC62797
3-Oxo-4,6-choladien-24-oic acid
3-Oxo-4,6-choladien-24-oic acid is an endogenous metabolite.
-
GC64220
3-Oxo-7-hydroxychol-4-enoic acid
3-Oxo-7-hydroxychol-4-enoic acid is an endogenous metabolite.
-
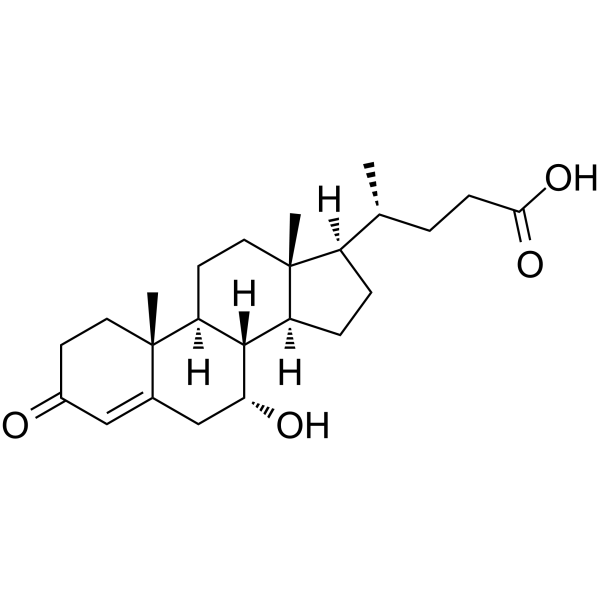
GC45339
3-Oxocholic Acid
A secondary bile acid
-
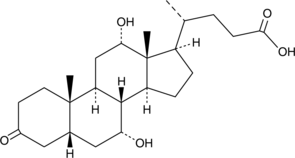
GC30353
3-Oxopentanedioic acid
3-Oxopentanedioic acid is a simple dicarboxylic acid, which is well-known to be used in the tropinone synthesis.
-
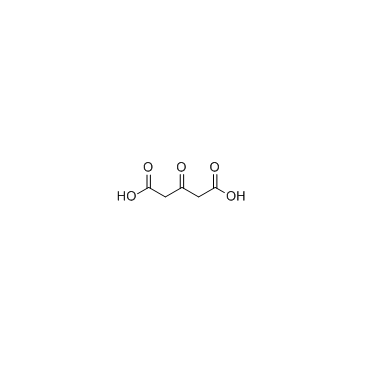
GC62798
3-Phenylbutyric acid
3-Phenylbutyric acid is metabolized by initial oxidation of the benzene ring and by initial oxidation of the side chain.
-
GC61582
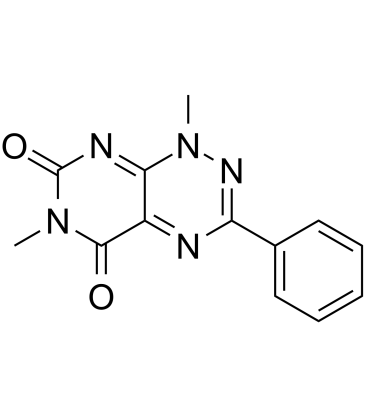
3-Phenyltoxoflavin
3-Phenyltoxoflavin, a derivative of Toxoflavin, is an Hsp90 inhibitor, with a Kd of 585 nM for the interaction of Hsp90-TPR2A. 3-Phenyltoxoflavin has anti-cancer activity.
-
GC33604
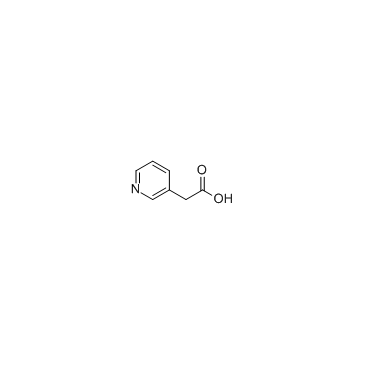
3-Pyridineacetic acid
3-Pyridineacetic acid is a higher homologue of nicotinic acid, a breakdown product of nicotine (and other tobacco alkaloids).
-
GC31553
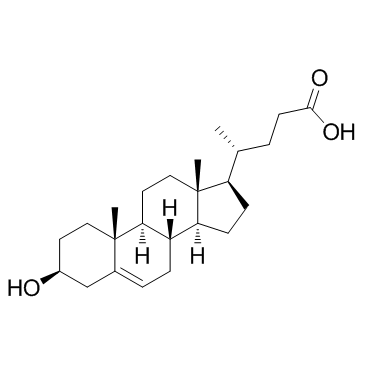
3b-Hydroxy-5-cholenoic acid
A bile acid
-
GC18817
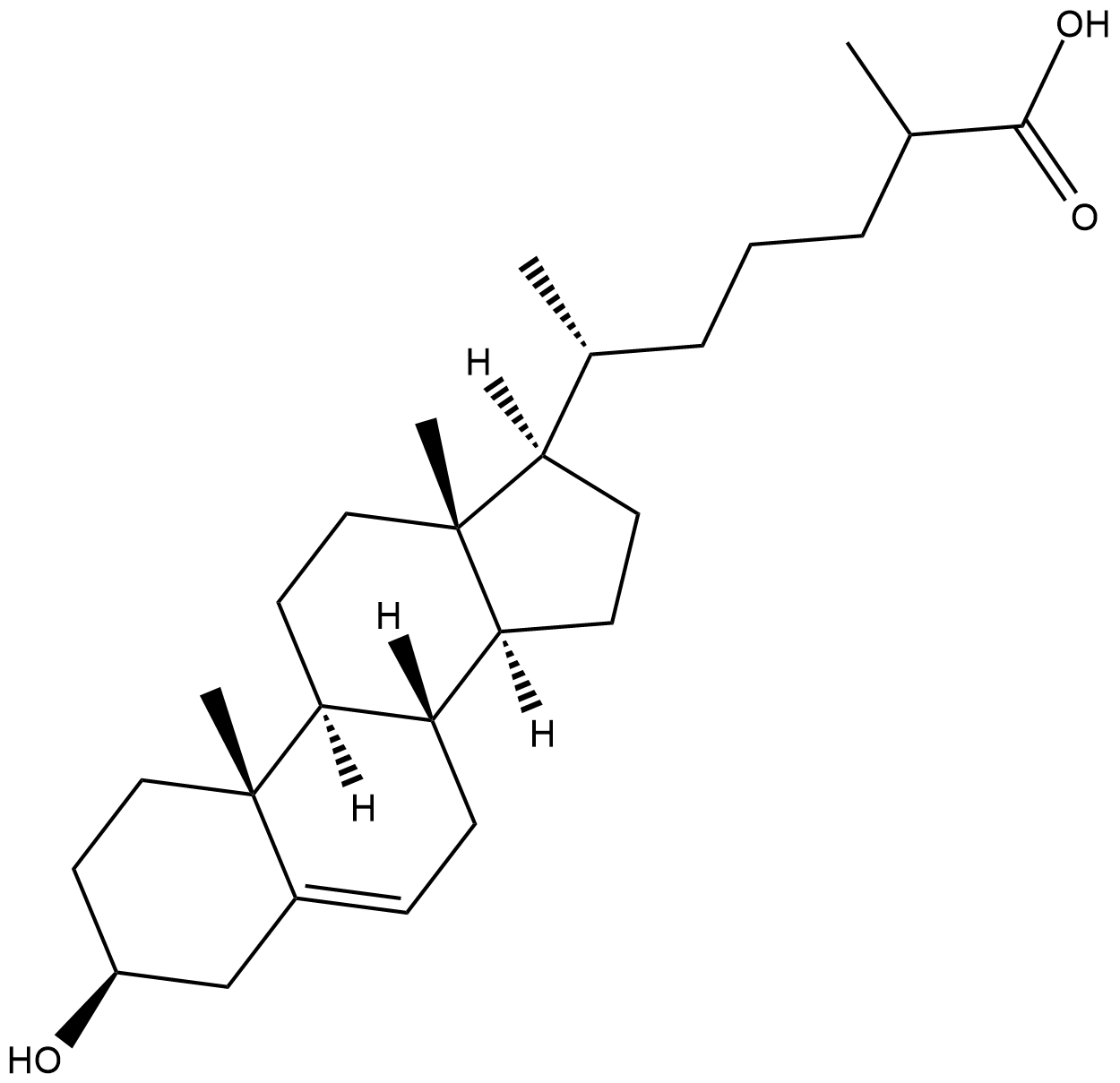
3β-hydroxy-5-Cholestenoic Acid
3β-hydroxy-5-Cholestenoic acid is an active metabolite of cholesterol formed when cholesterol is metabolized by the cytochrome P450 (CYP) isomer CYP27A1.
-
GC49367
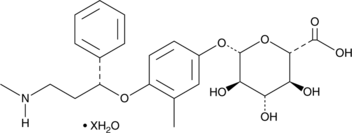
4’-hydroxy Atomoxetine Glucuronide (hydrate)
A metabolite of atomoxetine
-
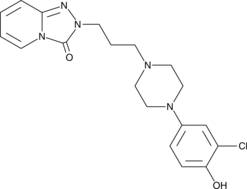
GC49252
4’-hydroxy Trazodone
A metabolite of trazodone
-
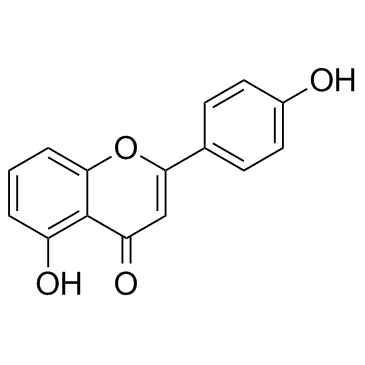
GC30277
4',5-Dihydroxyflavone
4',5-Dihydroxyflavone is a soybean LOX-1 and yeast α-Glucosidase inhibitor, with an Ki of 102.6 μM for soybean LOX-1 and an IC50 of 66 μM for yeast α-glucosidase.
-
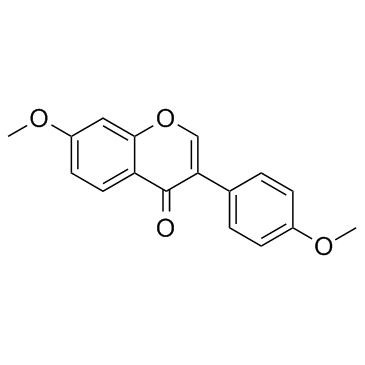
GC33976
4',7-Dimethoxyisoflavone (Dimethoxydaidzein)
4',7-Dimethoxyisoflavone (Dimethoxydaidzein) is isolated from the leaves of Albizzia lebbeck, which shows antifungal activity.
-
GC18527
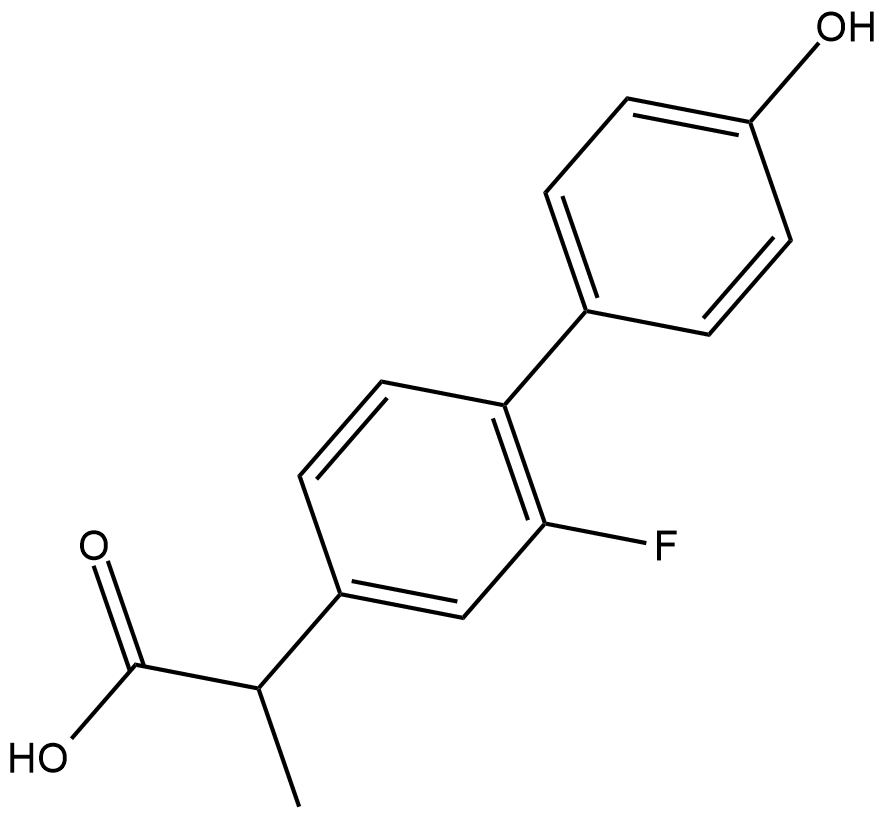
4'-hydroxy Flurbiprofen
A major active metabolite of flurbiprofen
-
GC41003
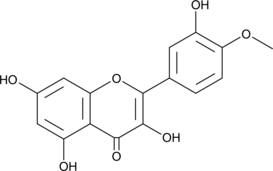
4'-O-methyl Quercetin
4'-O-methyl Quercetin (4'-O-Methyl Quercetin) is a natural flavonoid derivative of quercetin, with anti-oxidative and anti-inflammatory effects.
-
GC62800
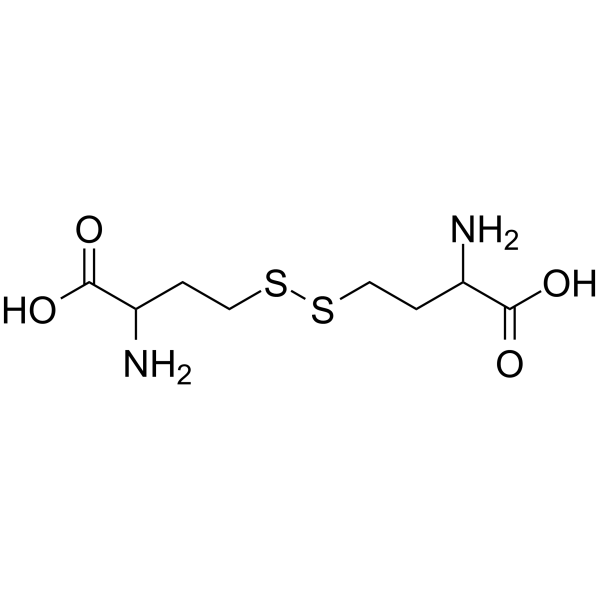
4,4’-Disulfanediylbis(2-aminobutanoic acid)
4,4’-Disulfanediylbis(2-aminobutanoic acid) is an endogenous metabolite.
-
GC60510
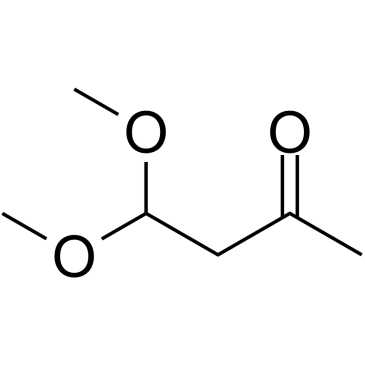
4,4-Dimethoxy-2-butanone
4,4-Dimethoxy-2-butanone is an endogenous metabolite.
-
GC49752
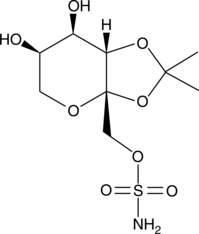
4,5-Desisopropylidene Topiramate
An inactive metabolite of topiramate
-
GC32483
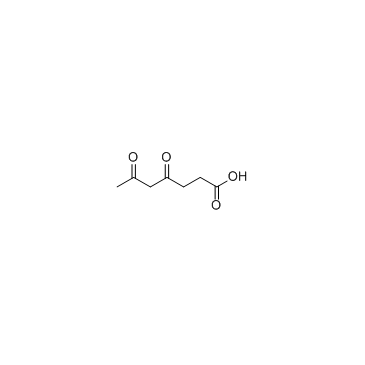
4,6-Dioxoheptanoic acid
4,6-Dioxoheptanoic acid is a potent inhibitor of heme biosynthesis.
-
GC31640
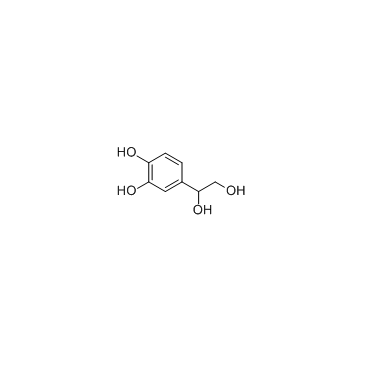
4-(1,2-Dihydroxyethyl)benzene-1,2-diol
4-(1,2-Dihydroxyethyl)benzene-1,2-diol, a normal norepinephrine metabolite, is found to be associated with Menkes syndrome.
-
GC49337
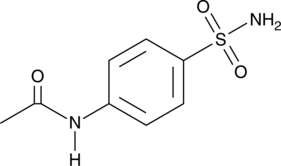
4-Acetamidobenzenesulfonamide
A metabolite of asulam and sulfanilamide
-
GC33504
4-Acetamidobutanoic acid (N-acetyl GABA)
4-Acetamidobutanoic acid (N-acetyl GABA) (N-acetyl GABA), the main metabolite of GABA, exhibits antioxidant and antibacterial activities.
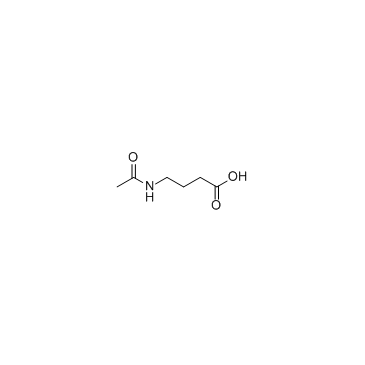
-
GC45341
4-Amino-6-chloro-1,3-benzenedisulfonamide
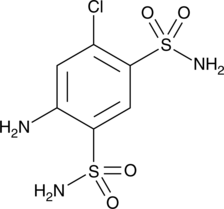
-
GC66484
4-Amino-L-phenylalanine hydrochloride
4-Amino-L-phenylalanine (H-Phe(4-NH2)-OH) hydrochloride is an endogenous metabolite.
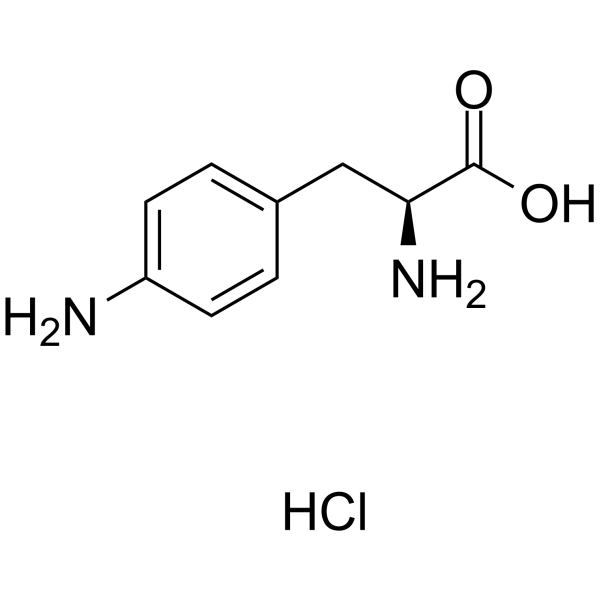
-
GC10996
4-Aminobenzoic acid
NULL
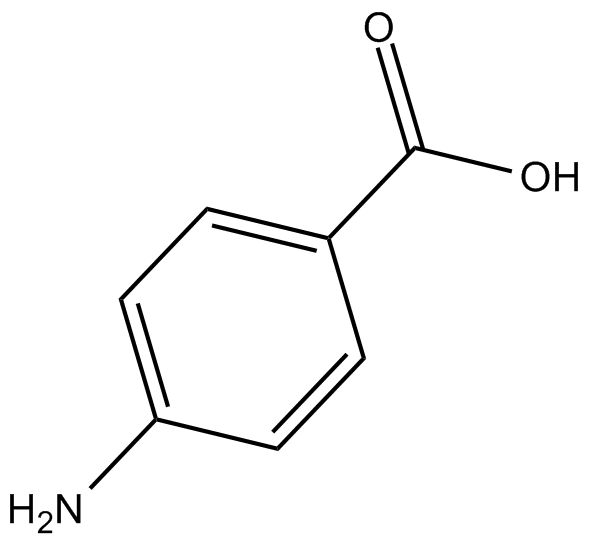
-
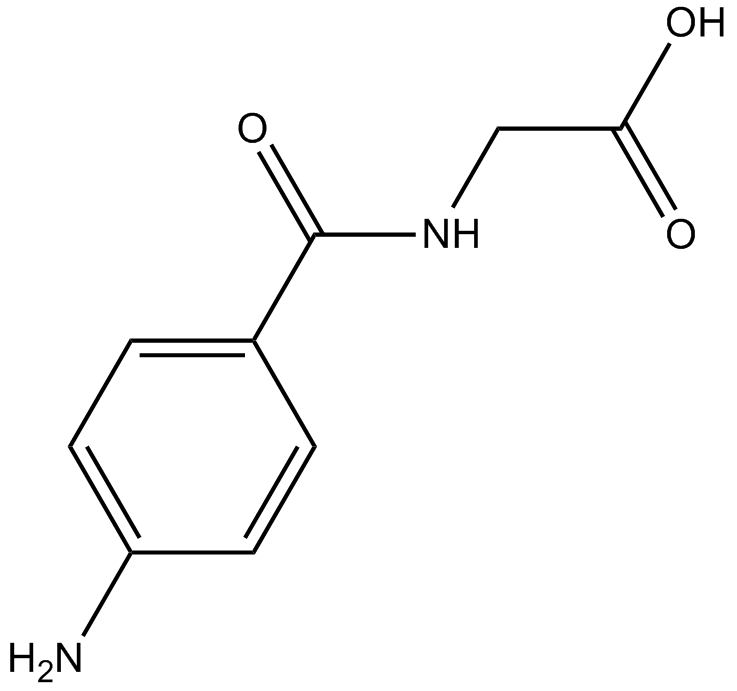
GC16443
4-Aminohippuric Acid
typical substrate of organic anion transport systems
-
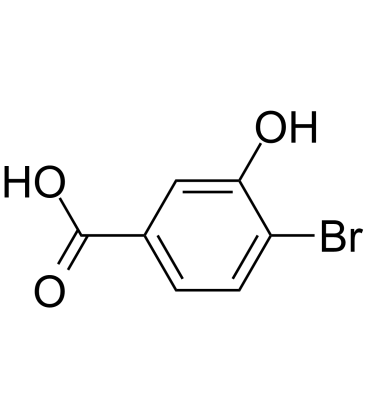
GC39473
4-Bromo-3-hydroxybenzoic acid
4-Bromo-3-hydroxybenzoic acid is a metabolite of Brocresine and a histidine decarboxylase (HDC) inhibitor with IC50s of 1 mM for both rat fetal and rat gastric HDC.
-
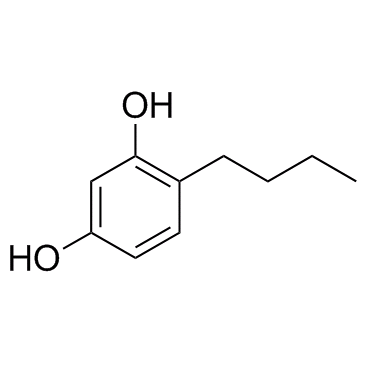
GC30513
4-Butylresorcinol (Butylresorcinol)
4-Butylresorcinol (Butylresorcinol) is a phenol derivative which can inhibit tyrosinase with IC50 of 11.27 μM.
-
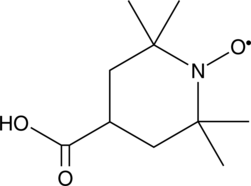
GC42351
4-carboxy TEMPO
4-carboxy TEMPO is a nitroxide and spin label.
-
GC60512
4-Carboxypyrazole
4-Carboxypyrazole is an endogenous metabolite.
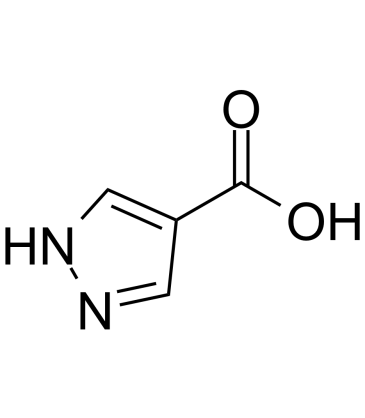
-
GC49722
4-CF3-TPP-DC
An inert mitochondriotropic carrier
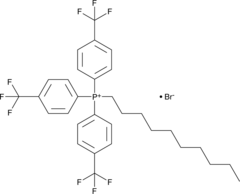
-
GC13337
4-Chlorophenylguanidine hydrochloride
Urokinase inhibitor, potent and specific
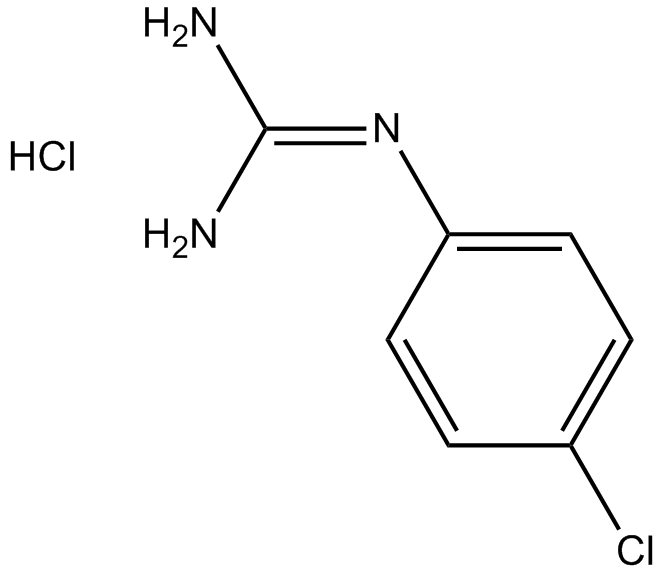
-
GC42370
4-desmethoxy Omeprazole
4-desmethoxy Omeprazole is a potential impurity found in commercial omeprazole and esomeprazole magnesium preparations.
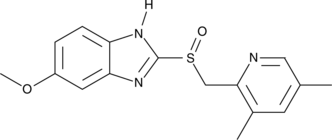
-
GC39245
4-Diethylaminobenzaldehyde
4-Diethylaminobenzaldehyde is a reversible aldehyde dehydrogenases (ALDHs) inhibitor, with a Ki of 4 nM for ALDH1. 4-Diethylaminobenzaldehyde displays potent anti-androgenic effect (IC50= 1.71μM).
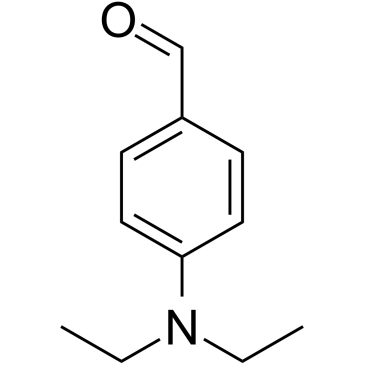
-
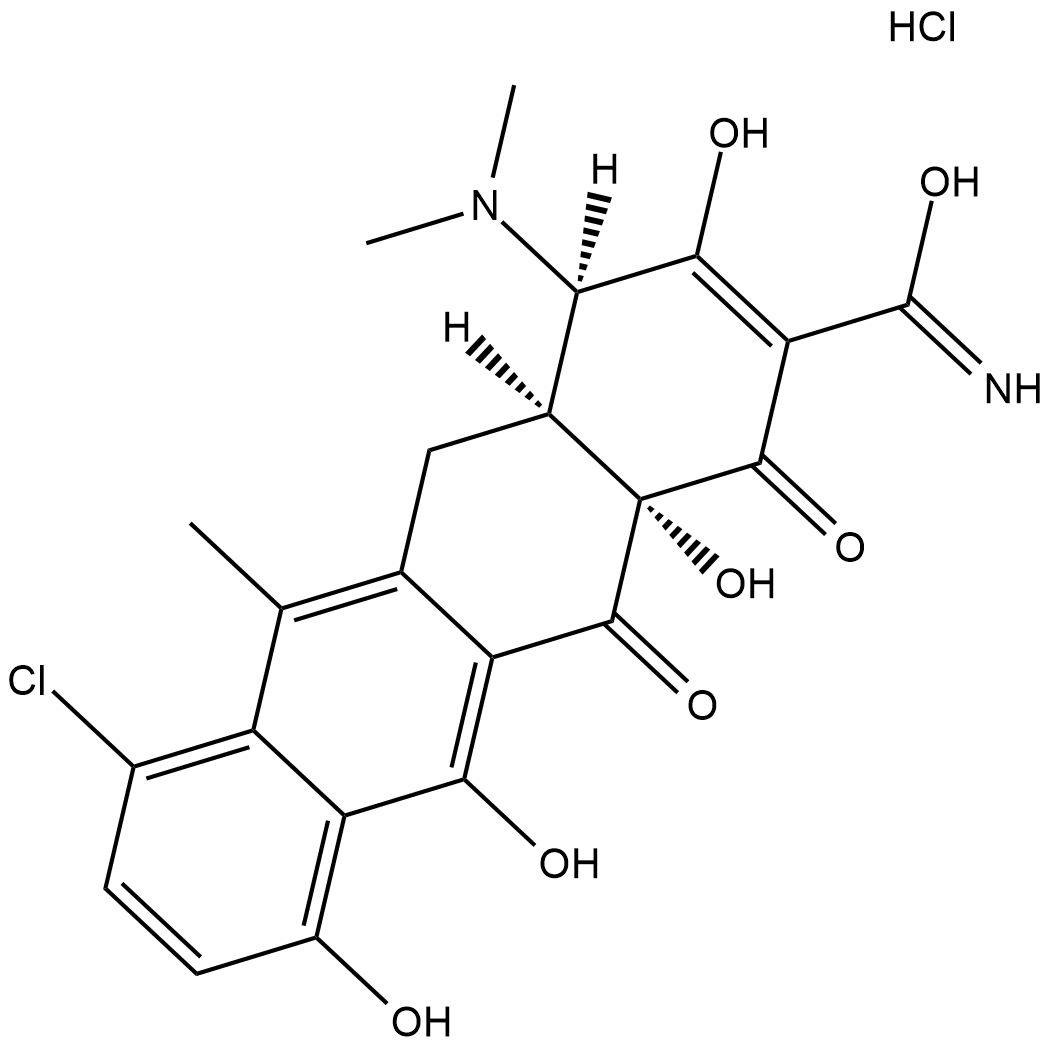
GC18359
4-Epianhydrochlortetracycline (hydrochloride)
4-Epianhydrochlortetracycline is a derivative of tetracycline .
-
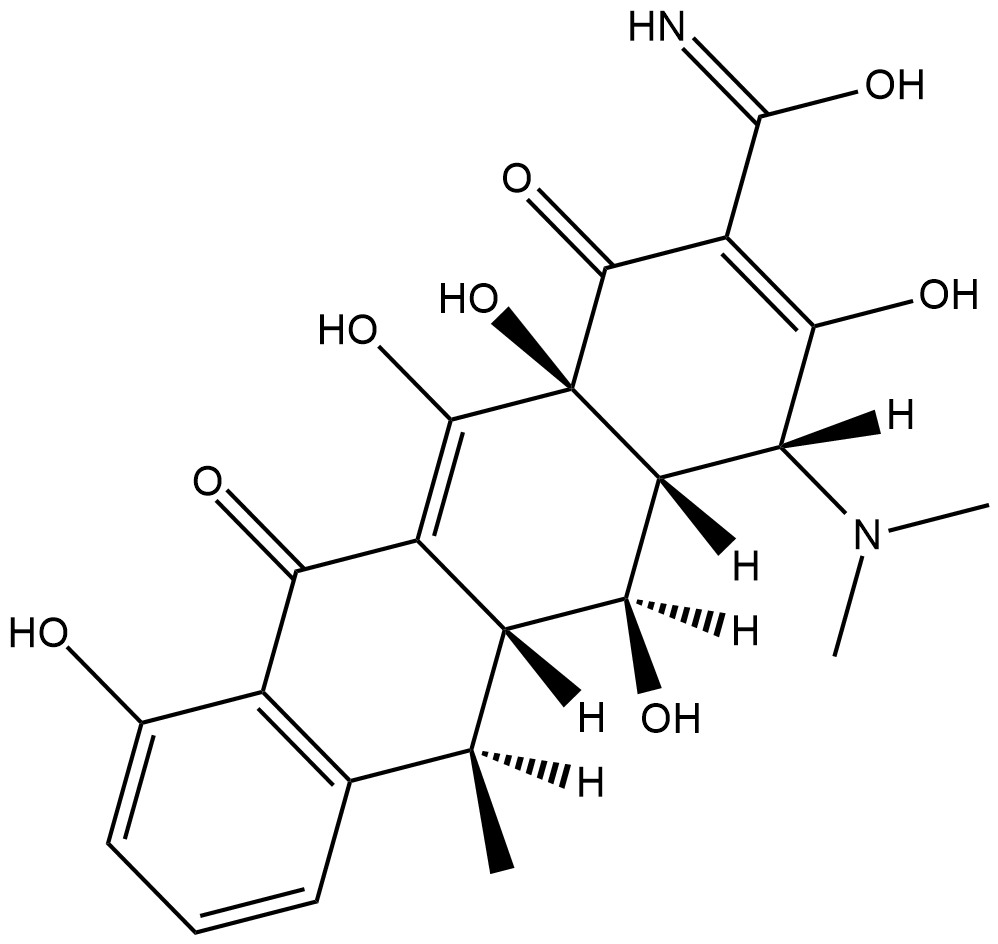
GC18194
4-Epidoxycycline
4-Epidoxycycline is the 4-epimer hepatic metabolite of the antibiotic doxycycline .
-
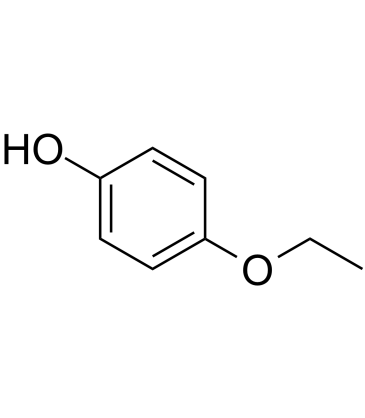
GC60514
4-Ethoxyphenol
4-Ethoxyphenol is an endogenous metabolite.
-
GC60515
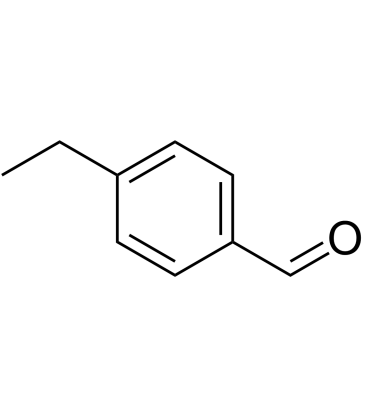
4-Ethylbenzaldehyde
-
GC30600
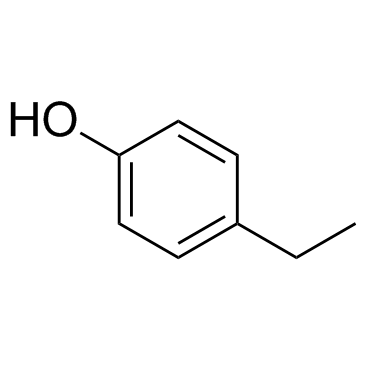
4-Ethylphenol
4-Ethylphenol is a volatile phenolic compound associated with off-odour in wine.
-
GC60516
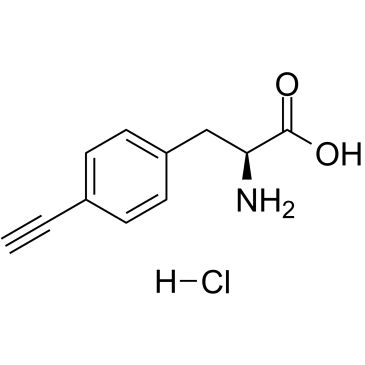
4-Ethynyl-L-phenylalanine hydrochloride
4-Ethynyl-L-phenylalanine hydrochloride (4-Ethynyl-L-phenylalanine hydrochloride) is a potent, selective, reversible and competitive inhibitor of tryptophan hydroxylase (TPH), with a Ki of 32.6 μM.
-
GC62803
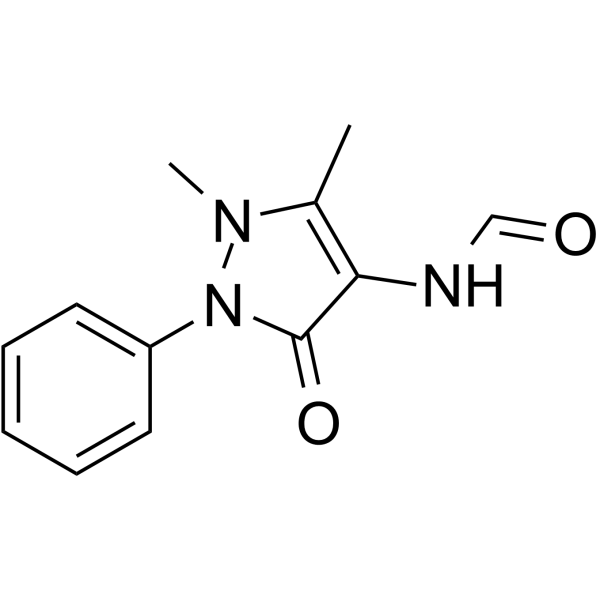
4-Formylaminoantipyrine
4-Formylaminoantipyrine?is an excreted metabolite of?aminophenazone.
-
GC30630
4-Guanidinobutanoic acid
4-Guanidinobutanoic acid is a normal metabolite present in low concentrations.
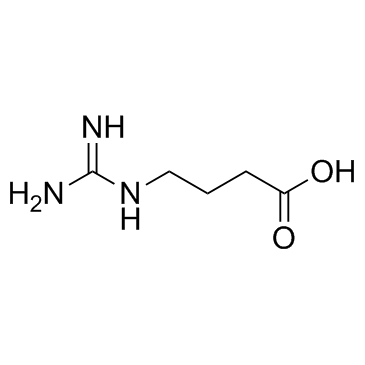
-
GC40097
4-HOBA
4-HOBA is an endogenous metabolite.
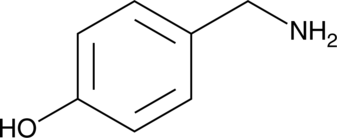
-
GC42401
4-hydroperoxy Cyclophosphamide
An activated analog of cyclophosphamide
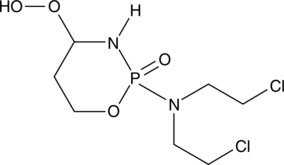
-
GC42405
4-hydroxy Atorvastatin (calcium salt)
4-hydroxy Atorvastatin is a metabolite of atorvastatin, an HMG-CoA reductase inhibitor present in formulations that have been used to treat hypercholesterolemia and certain dyslipidemias.
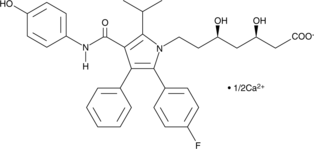
-
GC18425
4-hydroxy Atorvastatin lactone
4-hydroxy Atorvastatin lactone is a metabolite of atorvastatin , an HMG-CoA reductase inhibitor present in formulations that have been used to treat hypercholesterolemia and certain dyslipidemias.

-
GC42407
4-hydroxy Diclofenac
4-hydroxy Diclofenac is a CYP2C9 metabolite of the NSAID diclofenac.
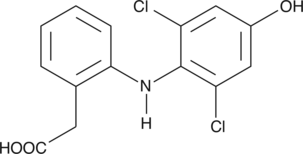
-
GC42410
4-hydroxy Nonenal
A lipid peroxidation product
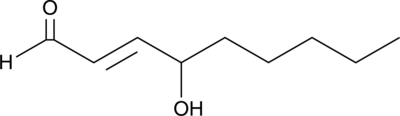
-
GC49575
4-hydroxy Omeprazole sulfide
A metabolite of omeprazole
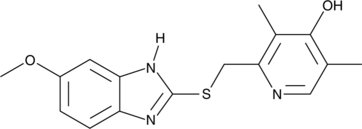
-
GC40879
4-hydroxy Solifenacin N-oxide
4-hydroxy Solifenacin N-oxide is an N-oxide form of 4-hydroxy solifenacin, the major metabolite of solifenacin .
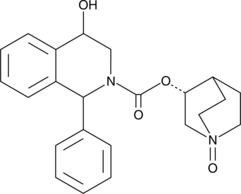
-
GC42415
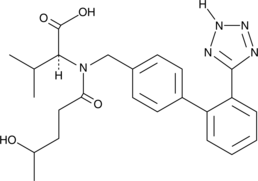
4-hydroxy Valsartan
4-hydroxy Valsartan is a major metabolite of the angiotensin II type 1 (AT1) receptor antagonist valsartan.
-
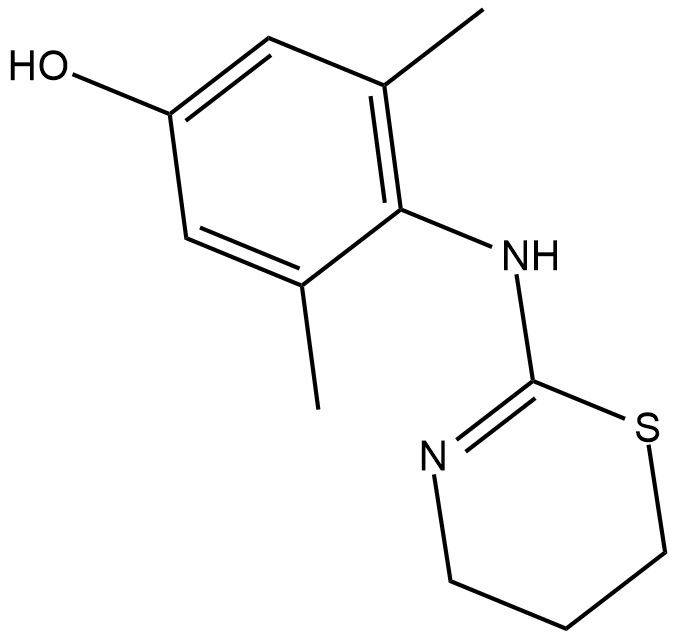
GC18417
4-hydroxy Xylazine
4-hydroxy Xylazine is a metabolite of xylazine .
-
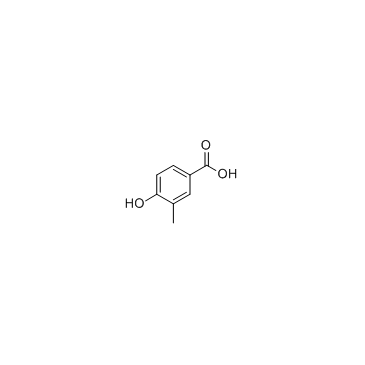
GC33656
4-Hydroxy-3-methylbenzoic acid
4-Hydroxy-3-methylbenzoic acid is a normal organic acid identified in urine specimens from a healthy population.
-
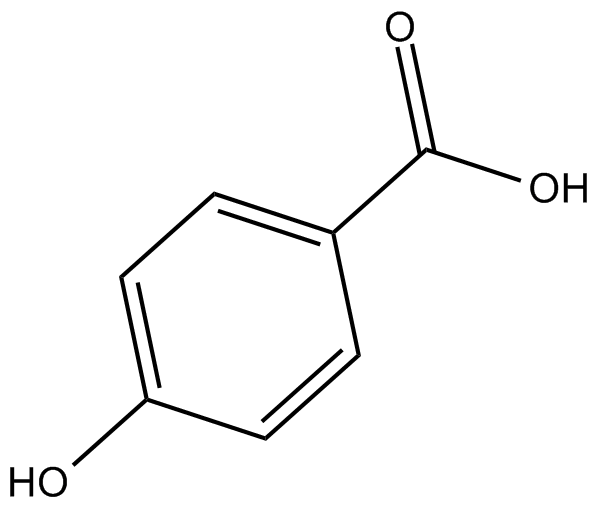
GC11270
4-Hydroxybenzoic acid
A phenolic acid
-
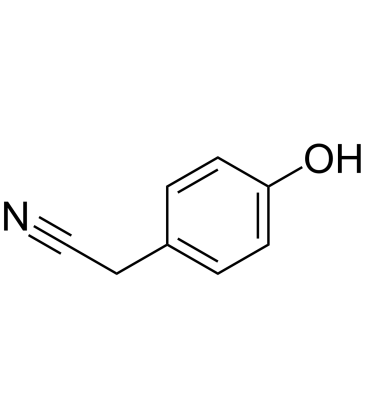
GC60518
4-Hydroxybenzyl cyanide
-
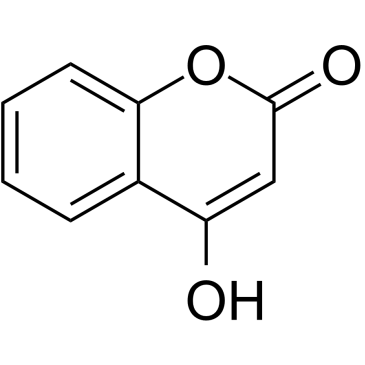
GC35131
4-Hydroxycoumarin
A coumarin
-
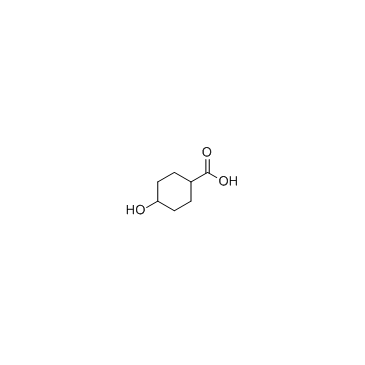
GC33610
4-Hydroxycyclohexanecarboxylic acid
4-Hydroxycyclohexanecarboxylic acid belongs to the class of organic compounds known as cyclohexanols.
-
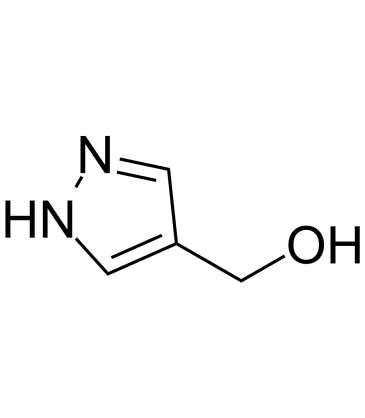
GC39691
4-Hydroxymethylpyrazole
4-Hydroxymethylpyrazole is the primary metabolite of Fomepizole.
-
GC68055
4-Hydroxyphenyl acetate

-
GC33815
4-Hydroxyphenylacetic acid
A phenolic acid with anti-inflammatory activity
-
GC35134
4-Methoxybenzaldehyde
4-Methoxybenzaldehyde is a naturally occurring fragrant phenolic compound.
-
GC35135
4-Methoxycinnamic acid
4-Methoxycinnamic acid is detected as natural phenylpropanoid in A.
-
GC60521
4-Methyl-1-phenyl-2-pentanone
4-Methyl-1-phenyl-2-pentanone is an endogenous metabolite.
-
GC31307
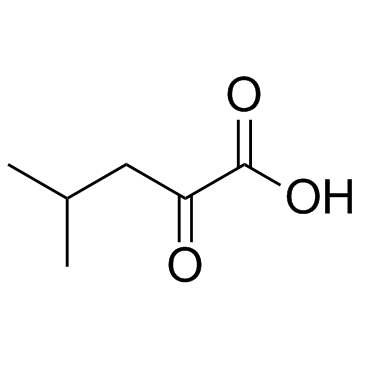
4-Methyl-2-oxopentanoic acid
4-Methyl-2-oxopentanoic acid (α-Ketoisocaproic acid), an abnormal metabolite, is both a neurotoxin and a metabotoxin.
-
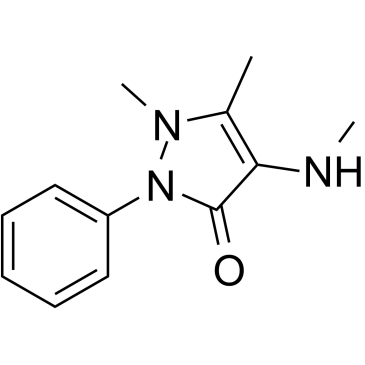
GC39307
4-Methylamino antipyrine
4-Methylamino antipyrine is an active metabolite of Metamizole.
-
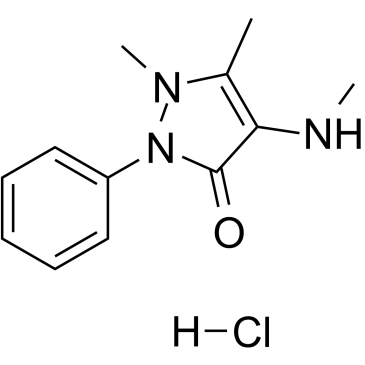
GC60523
4-Methylamino antipyrine hydrochloride
An active metabolite of metamizole
-
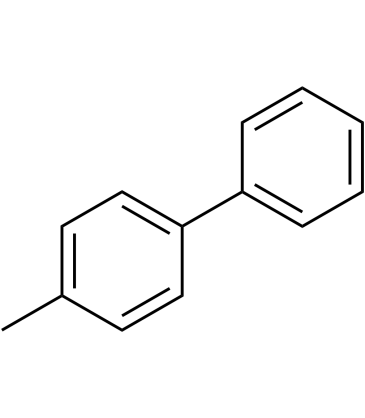
GC39780
4-Methylbiphenyl
-
GC30658
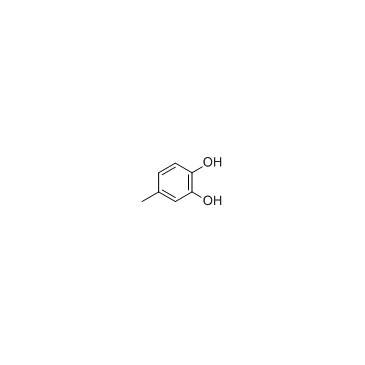
4-Methylcatechol
4-Methylcatechol, a metabolite of p-toluate, is a substrate as well as a suicide inhibitor of Catechol 2,3-Dioxygenase.
-
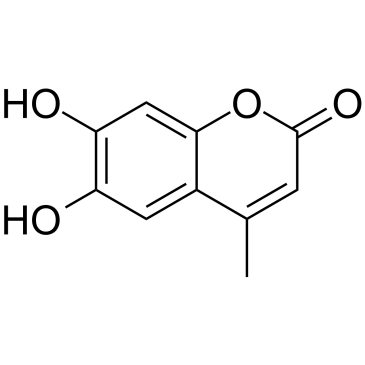
GC35139
4-Methylesculetin
4-Methylesculetin is an orally active natural coumarin derivative, with potent anti-oxidant and anti-inflammatory activities.
-
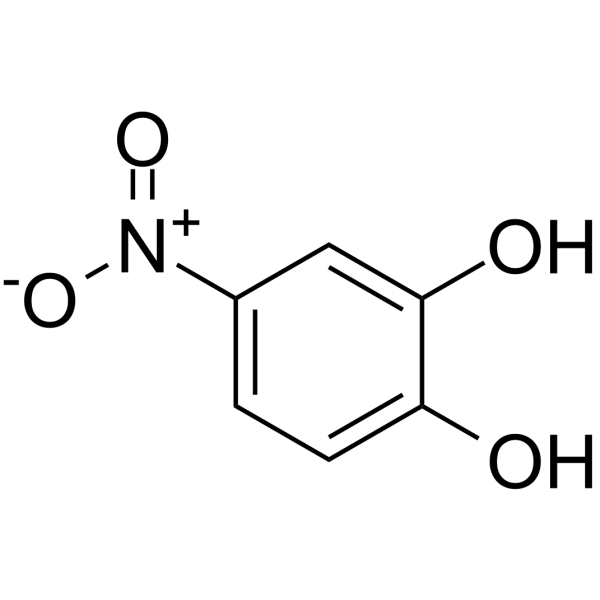
GC64621
4-Nitrocatechol
4-Nitrocatechol is a potent lipoxygenase inhibitor.
-
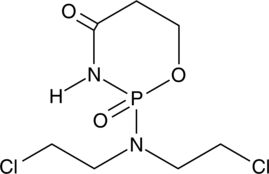
GC49127
4-oxo Cyclophosphamide
An inactive metabolite of cyclophosphamide
-
GC60524
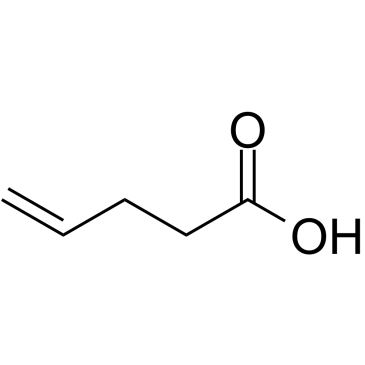
4-Pentenoic acid
4-Pentenoic acid is an endogenous metabolite.
-
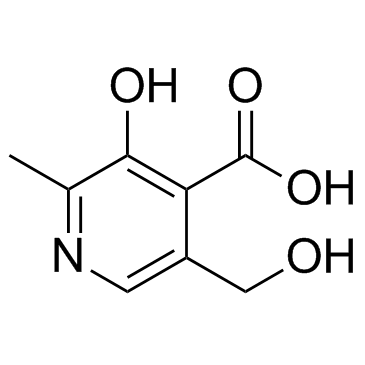
GC30670
4-Pyridoxic acid
A pyridoxine metabolite
-
GC60525
4-Vinylphenol (10%w/w in propylene glycol)
4-Vinylphenol is found in the medicinal herb Hedyotis diffusa Willd, wild rice and is also the metabolite of p-coumaric and ferulic acid by lactic acid bacteria in wine. 4-Vinylphenol induces apoptosis and inhibits blood vessels formation and suppresses invasive breast tumor growth in vivo.
-
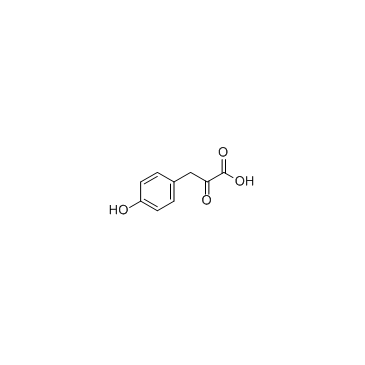
GC35126
4-Hydroxyphenylpyruvic acid
4-Hydroxyphenylpyruvic acid is an intermediate in the metabolism of the amino acid phenylalanine.
-
GC60531
5α-Cholestan-3-one
5α-Cholestan-3-one is an endogenous metabolite.
-
GC41399
5α-dihydro Levonorgestrel
5α-dihydro Levonorgestrel is a metabolite of the synthetic progestin levonorgestrel.
-
GC30669
5α-Cholestan-3β-ol (5α-Cholestanol)
5α-Cholestan-3β-ol (5α-Cholestanol) is a derivitized steroid compound.
-
GC38358
5'-Cytidylic acid
5'-Cytidylic acid (5'-Cytidylic acid) is a nucleotide which is used as a monomer in RNA.
-
GC33636
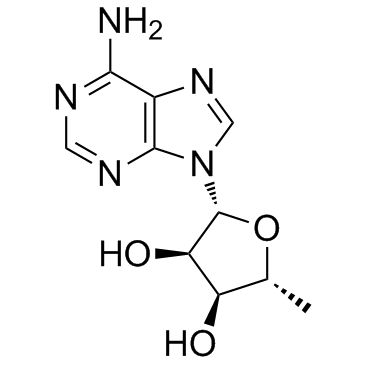
5'-Deoxyadenosine
An adenosine analog
-
GC35157
5'-GTP trisodium salt
5'-GTP trisodium salt (5'-GTP trisodium salt) is an activator of the signal transducing G proteins which are involved in various cellular processes including proliferation, differentiation, and activation of several intracellular kinase cascades.
-
GC35159
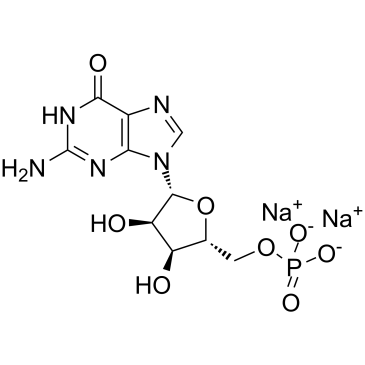
5'-Guanylic acid disodium salt
5'-Guanylic acid disodium salt (5'-GMP disodium salt) is composed of guanine, ribose, and phosphate moieties and it is a nucleotide monomer in messenger RNA.
-
GC18582
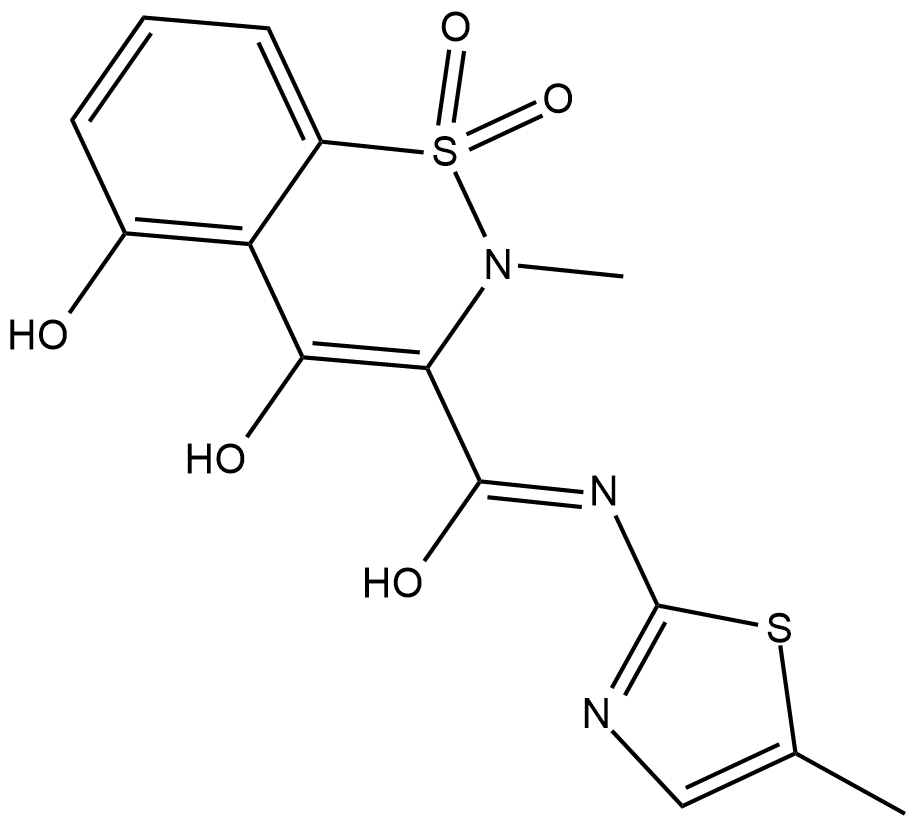
5'-hydroxy Meloxicam
A metabolite of meloxicam
-
GC40459
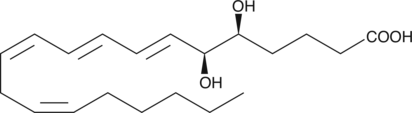
5(R)-HETE
5(R)-HETE is a rare lipoxygenase product of arachidonic acid.
-
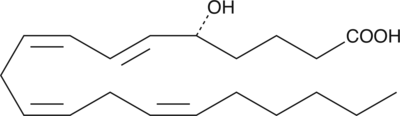
GC41126
5(S),12(S)-DiHETE
5(S),12(S)-DiHETE is a natural bioactive lipid derived from arachidonic acid.
-
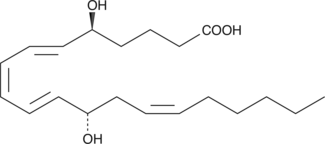
GC41127
5(S),15(S)-DiHETE
5(S),15(S)-DiHETE is synthesized by 15-LO from 5(S)-HETE.
-
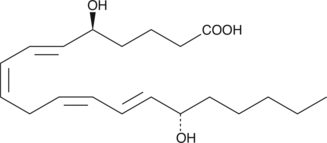
GC41128
5(S),6(R)-11-trans DiHETE
5(S),6(R)-11-trans DiHETE is a C-11 double bond isomer of 5(S),6(R)-DiHETE that is formed by the enzymatic isomerization of 5(S),6(R)-DiHETE by a membrane bound factor.
-
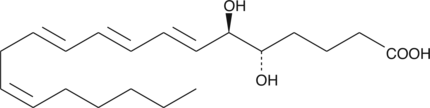
GC41129
5(S),6(R)-DiHETE
5(S),6(R)-DiHETE is a dihydroxy polyunsaturated fatty acid and a nonenzymatic hydrolysis product of leukotriene A4 (LTA4).
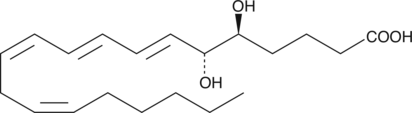
-
GC41131
5(S),6(S)-DiHETE
5(S),6(S)-DiHETE is one of the four diastereomeric 5,6-dihydroxy acids produced from the non-enzymatic hydrolysis of LTA4.