Proteases
Proteases is a general term for a class of enzymes that hydrolyze protein peptide chains. According to the way they degrade polypeptides, they are divided into two categories: endopeptidases and telopeptidases. The former can cut the large molecular weight polypeptide chain from the middle to form prions and peptones with smaller molecular weights; the latter can be divided into carboxypeptidase and aminopeptidase, which respectively remove the peptide from the free carboxyl terminus or free amino terminus of the polypeptide one by one. Chain hydrolysis produces amino acids.
A general term for a class of enzymes that hydrolyze peptide bonds in proteins. According to the way they hydrolyze polypeptides, they can be divided into endopeptidases and exopeptidases. Endopeptidase cleaves the interior of the protein molecule to form smaller molecular weight peptones and peptones. Exopeptidase hydrolyzes peptide bonds one by one from the end of the free amino group or carboxyl group of protein molecules, and frees amino acids, the former is aminopeptidase and the latter is carboxypeptidase. Proteases can be classified into serine proteases, sulfhydryl proteases, metalloproteases and aspartic proteases according to their active centers and optimum pH. According to the optimum pH value of its reaction, it is divided into acidic protease, neutral protease and alkaline protease. The proteases used in industrial production are mainly endopeptidases.
Proteases are widely found in animal offal, plant stems and leaves, fruits and microorganisms. Microbial proteases are mainly produced by molds and bacteria, followed by yeast and actinomycetes.
Enzymes that catalyze the hydrolysis of proteins. There are many kinds, the important ones are pepsin, trypsin, cathepsin, papain and subtilisin. Proteases have strict selectivity for the reaction substrates they act on. A protease can only act on certain peptide bonds in protein molecules, such as the peptide bonds formed by the hydrolysis of basic amino acids catalyzed by trypsin. Proteases are widely distributed, mainly in the digestive tract of humans and animals, and are abundant in plants and microorganisms. Due to limited animal and plant resources, the industrial production of protease preparations is mainly prepared by fermentation of microorganisms such as Bacillus subtilis and Aspergillus terrestris.
Targets for Proteases
- Caspase(114)
- Aminopeptidase(24)
- ACE(74)
- Calpains(20)
- Carboxypeptidase(10)
- Cathepsin(81)
- DPP-4(31)
- Elastase(26)
- Gamma Secretase(67)
- HCV Protease(59)
- HSP(113)
- HIV Integrase(37)
- HIV Protease(47)
- MMP(228)
- NS3/4a protease(8)
- Serine Protease(18)
- Thrombin(58)
- Urokinase(4)
- Cysteine Protease(0)
- Other Proteases(18)
- Tyrosinases(47)
- 15-PGDH(1)
- Acetyl-CoA Carboxylase(13)
- Acyltransferase(59)
- Aldehyde Dehydrogenase (ALDH)(28)
- Aminoacyl-tRNA Synthetase(9)
- ATGL(1)
- Dipeptidyl Peptidase(56)
- Drug Metabolite(457)
- E1/E2/E3 Enzyme(90)
- Endogenous Metabolite(1636)
- FABP(30)
- Farnesyl Transferase(23)
- Glutaminase(14)
- Glutathione Peroxidase(14)
- Isocitrate Dehydrogenase (IDH)(28)
- Lactate Dehydrogenase(17)
- Lipoxygenase(234)
- Mitochondrial Metabolism(207)
- NEDD8-activating Enzyme(7)
- Neprilysin(12)
- PAI-1(13)
- Ser/Thr Protease(41)
- Tryptophan Hydroxylase(13)
- Xanthine Oxidase(18)
- MALT1(10)
- PCSK9(1)
Products for Proteases
- Cat.No. Product Name Information
-
GC40378
5(S)-HEPE
5(S)-HEPE is produced by 5-lipoxygenase catalyzed oxidation of eicosapentaenoic acid (EPA).
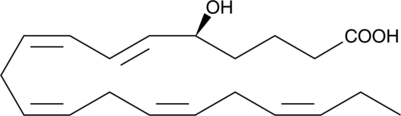
-
GC40460
5(S)-HETE
5(S)-HETE is produced by the action of 5-LO on arachidonic acid to give 5(S)-HpETE, followed by reduction of the hydroperoxide.
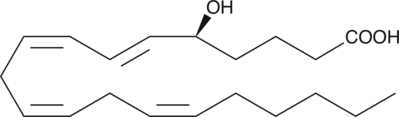
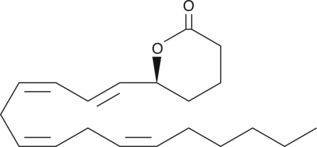
-
GC40829
5(S)-HETE lactone
5(S)-HETE lactone is a cyclic ester formed by acid-catalyzed nucleophilic addition of the C-5 hydroxyl to the C-1 carboxyl of 5(S)-HETE.
-
GC46679
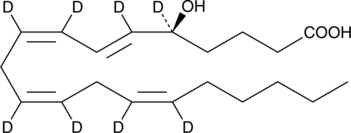
5(S)-HETE-d8
An internal standard for the quantification of 5-HETE
-
GC42479
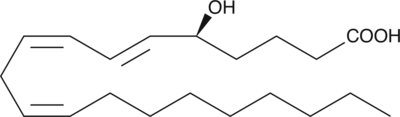
5(S)-HETrE
5(S)-HETrE is produced by the action of 5-LO when mead acid is the substrate.
-
GC41105
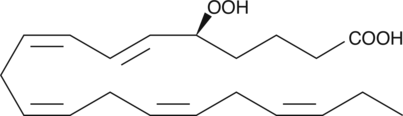
5(S)-HpEPE
5(S)-HpEPE is a monohydroperoxy polyunsaturated fatty acid produced by the action of 5-LO on EPA.
-
GC40784
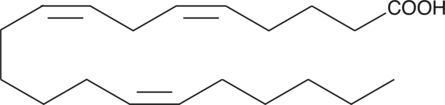
5(Z),8(Z),14(Z)-Eicosatrienoic Acid
5(Z),8(Z),14(Z)-Eicosatrienoic acid is a polyunsaturated fatty acid that can be a substrate for 5-lipoxygenase (5-LO).
-
GC40830
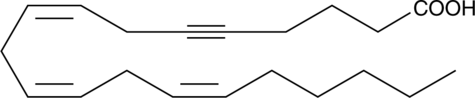
5,6-dehydro Arachidonic Acid
5,6-dehydro Arachidonic acid is an analog of arachidonic acid with an acetylene in the 5,6 position.
-
GC18542
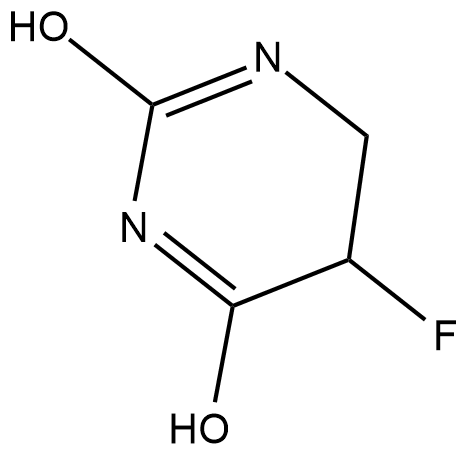
5,6-dihydro-5-Fluorouracil
5,6-dihydro-5-Fluorouracil (5-FUH2) is formed by the hydrogenation of 5-fluorouracil via the enzyme dihydropyrimidine dehydrogenase (DPD).
-
GC35152
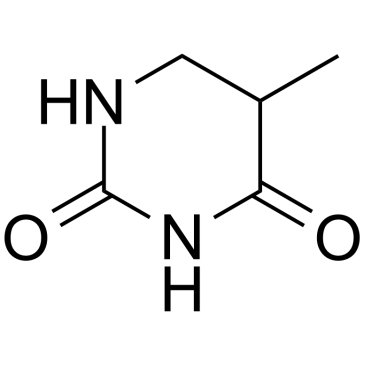
5,6-Dihydro-5-methyluracil
5,6-Dihydro-5-methyluracil (Dihydrothymine), an intermediate breakdown product of thymine, comes from animal or plants.
-
GC33650
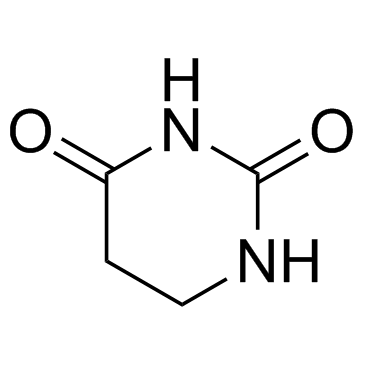
5,6-Dihydrouracil
5,6-Dihydrouracil (5,6-5,6-Dihydrouracil), a metabolite of Uracil, can be used as a marker for identification of dihydropyrimidine dehydrogenase (DPD)-deficient.
-
GC33502
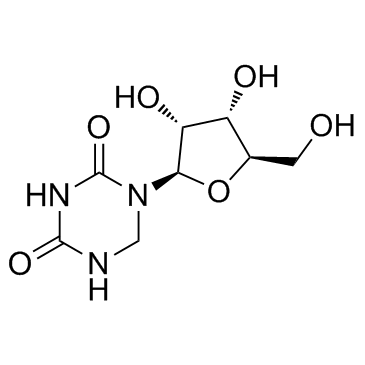
5,6-Dihydrouridine
A pyrimidine nucleoside and derivative of uridine
-
GC42484
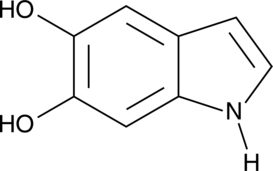
5,6-dihydroxy Indole
5,6-dihydroxy Indole is an intermediate in melanogenesis.
-
GC38286
5,6-Dimethyl-1H-benzo[d]imidazole
5,6-Dimethyl-1H-benzo[d]imidazole is an endogenous metabolite.
![5,6-Dimethyl-1H-benzo[d]imidazole Chemical Structure 5,6-Dimethyl-1H-benzo[d]imidazole Chemical Structure](/media/struct/GC3/GC38286.png)
-
GC18466
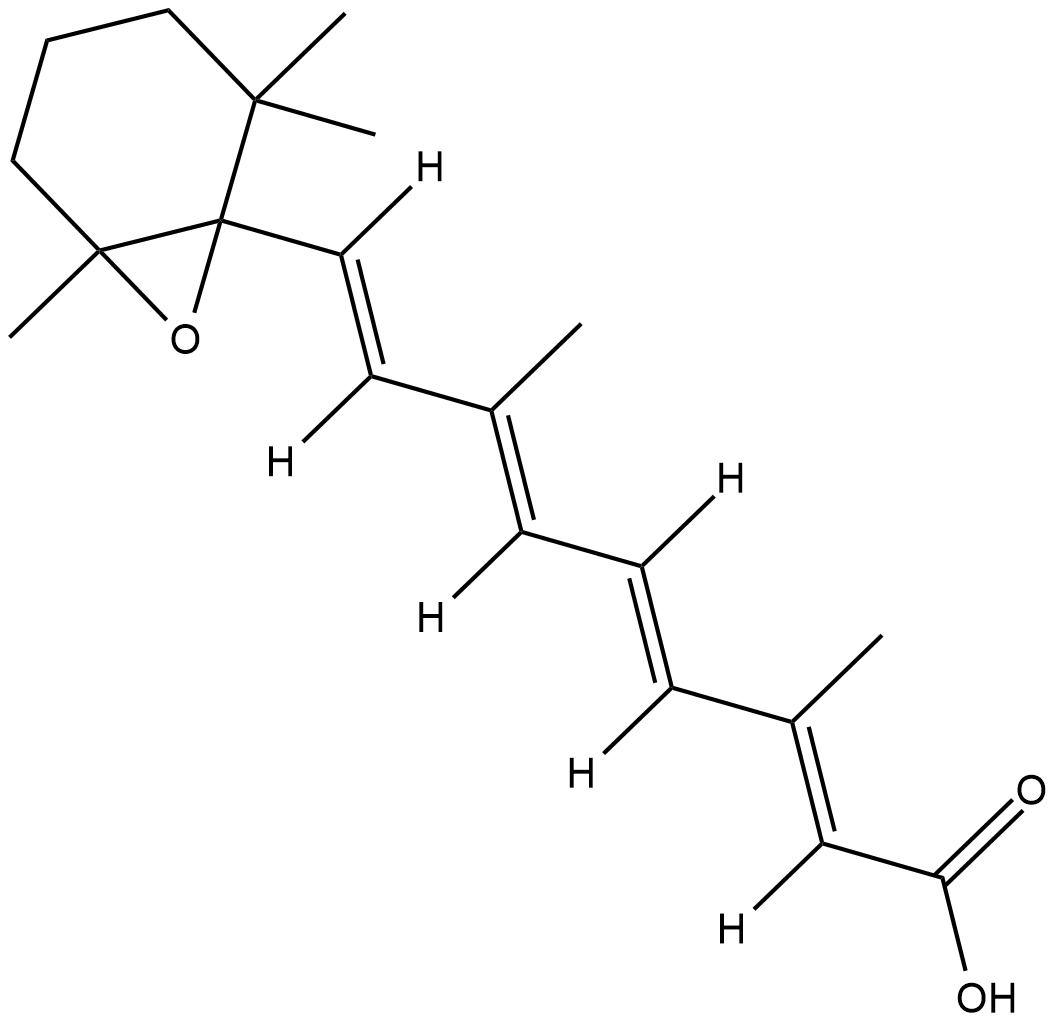
5,6-epoxy-13-cis Retinoic Acid
5,6-epoxy-13-cis Retinoic acid is a metabolite of 13-cis retinoic acid.
-
GC35150
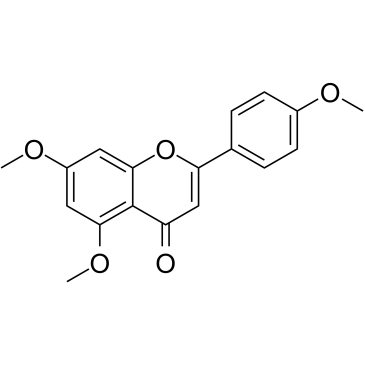
5,7,4'-Trimethoxyflavone
5,7,4'-Trimethoxyflavone is isolated from Kaempferia parviflora (KP) that is a famous medicinal plant from Thailand. 5,7,4'-Trimethoxyflavone induces apoptosis, as evidenced by increments of sub-G1 phase, DNA fragmentation, annexin-V/PI staining, the Bax/Bcl-xL ratio, proteolytic activation of caspase-3, and degradation of poly (ADP-ribose) polymerase (PARP) protein.5,7,4'-Trimethoxyflavone is significantly effective at inhibiting proliferation of SNU-16 human gastric cancer cells in a concentration dependent manner.
-
GN10629
5,7-dihydroxychromone
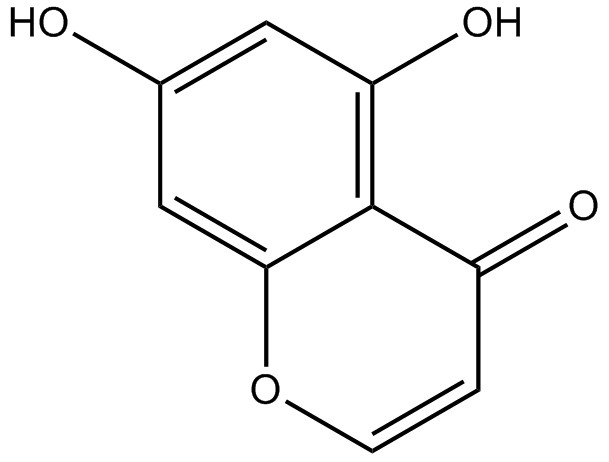
-
GC52227
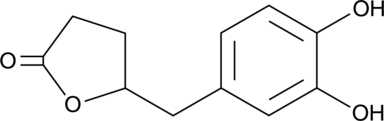
5-(3',4'-Dihydroxyphenyl)-γ-Valerolactone
An active metabolite of various polyphenols
-
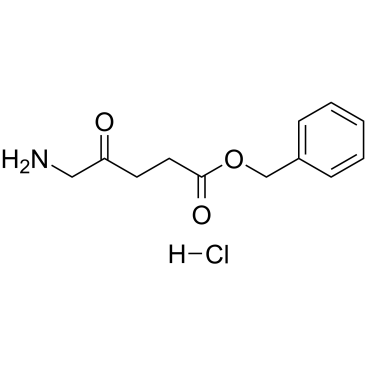
GC38882
5-ALA benzyl ester hydrochloride
5-ALA benzyl ester hydrochloride (Benzyl-ALA hydrochloride) is a protoporphyrin precursor used as a photodetection agent. 5-ALA benzyl ester hydrochloride induces protoporphyrin IX (PPIX) accumulation in colon carcinoma cell lines.
-
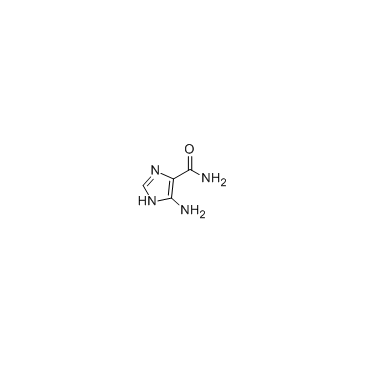
GC35156
5-Amino-3H-imidazole-4-Carboxamide
5-Amino-3H-imidazole-4-Carboxamide (AICA) is an important precursor for the synthesis of purines in general and of the nucleobases adenine and guanine in particular.
-
GC32608
5-Amino-4-oxopentanoic acid
5-Amino-4-oxopentanoic acid (5-ALA) is a non-protein amino acid that plays a rate-limiting role in heme biosynthesis.
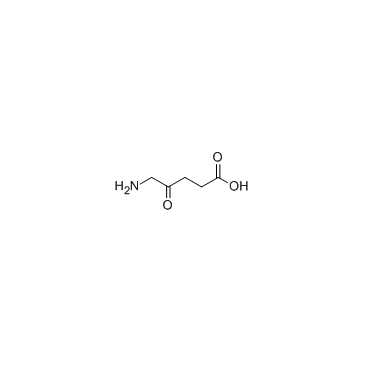
-
GC45356
5-Aminolevulinic Acid (hydrochloride)
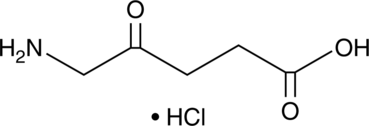
-
GC52413
5-Aminosalicylic Acid-d7
An internal standard for the quantification of 5-aminosalicylic acid
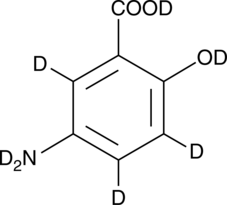
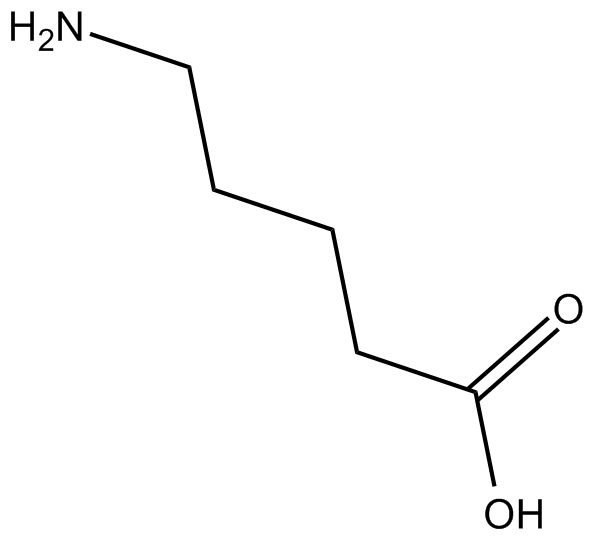
-
GC12713
5-Aminovaleric acid hydrochloride
5-Aminovaleric acid hydrochloride is believed to act as a methylene homologue of gamma-aminobutyric acid (GABA) and functions as a weak GABA agonist.
-
GN10062
5-HTP

-
GC49655
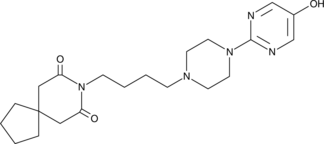
5-hydroxy Buspirone
A metabolite of buspirone
-
GC42546
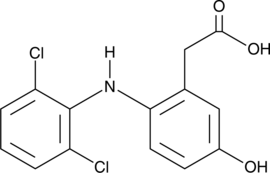
5-hydroxy Diclofenac
5-hydroxy Diclofenac is a metabolite of the NSAID diclofenac formed by the cytochrome P450 (CYP) isoform CYP3A4.
-
GC49119
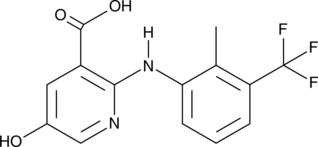
5-hydroxy Flunixin
A metabolite of flunixin
-
GC49315
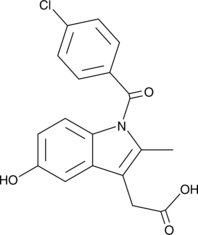
5-hydroxy Indomethacin
A metabolite of indomethacin
-
GC41312
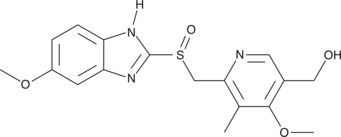
5-hydroxy Omeprazole
5-hydroxy Omeprazole is a major metabolite of omeprazole, an inhibitor of the gastric H+/K+-ATPase pump.
-
GC42549
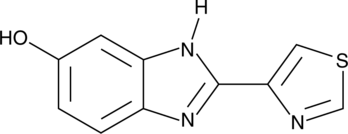
5-hydroxy Thiabendazole
5-hydroxy Thiabendazole (5-OH TBZ) is a major metabolite of the anthelmintic thiabendazole.
-
GC12829
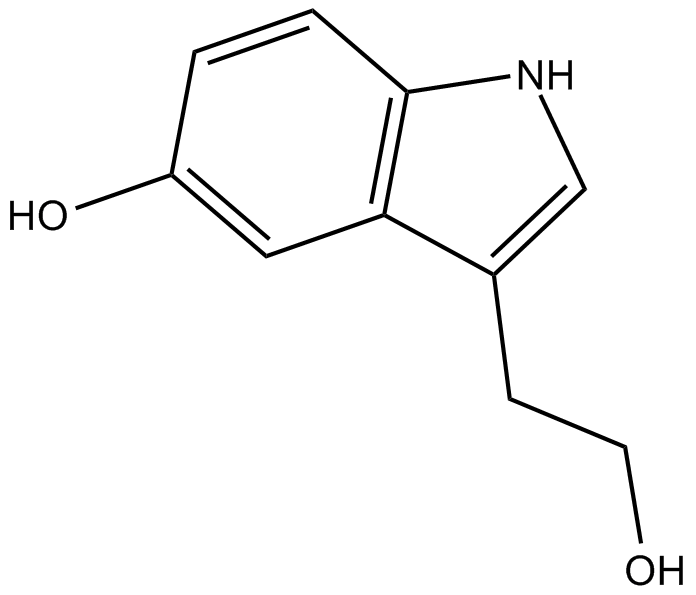
5-hydroxy Tryptophol
metabolite of tryptophan
-
GC60529
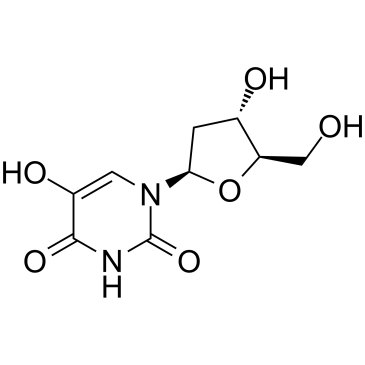
5-Hydroxy-2'-deoxyuridine
5-Hydroxy-2'-deoxyuridine (5-OHdU) is a major stable oxidation product of 2'-Deoxycytidine.
-
GC42550
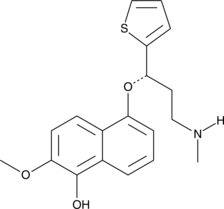
5-hydroxy-6-methoxy (S)-Duloxetine
5-hydroxy-6-methoxy (S)-Duloxetine is a metabolite of (S)-duloxetine.
-
GC30613
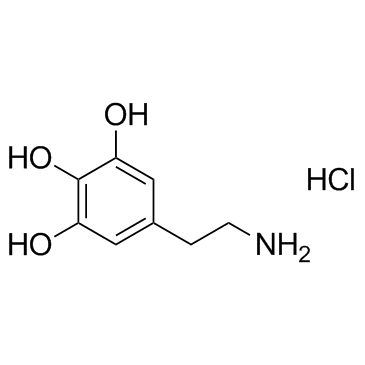
5-Hydroxydopamine hydrochloride
5-Hydroxydopamine is a naturally occurring amine in human urine.
-
GC33698
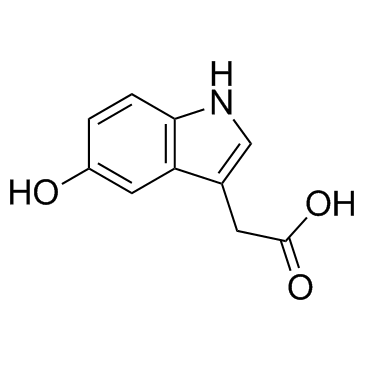
5-Hydroxyindole-3-acetic acid
A serotonin metabolite
-
GC42551
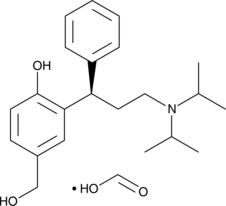
5-hydroxymethyl Tolterodine (formate)
5-hydroxymethyl Tolterodine is an active metabolite of the muscarinic acetylcholine receptor antagonists tolterodine and fesoterodine.
-
GC30189
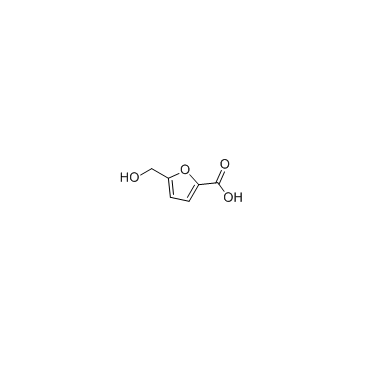
5-Hydroxymethyl-2-furancarboxylic acid
A major metabolite of 5-hydroxymethyl-2-furfural
-
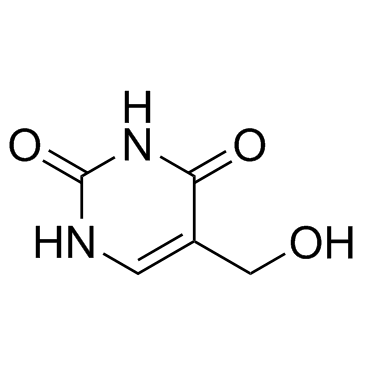
GC33613
5-Hydroxymethyluracil
Product of oxidative damage to DNA
-
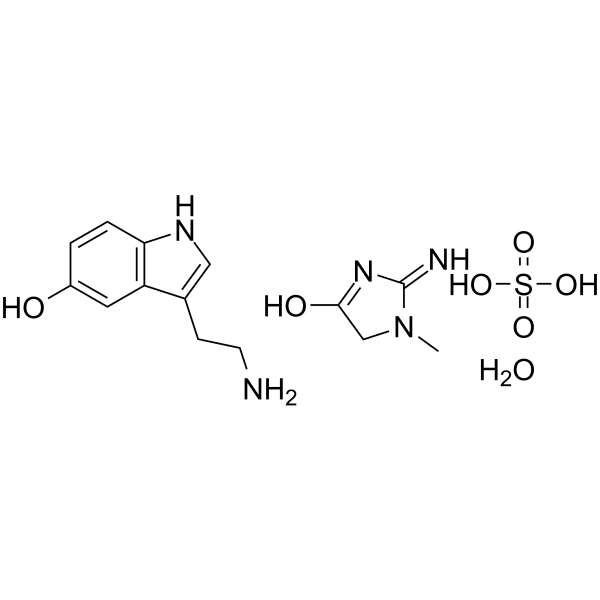
GC62810
5-Hydroxytryptamine creatinine sulfate monohydrate
5-Hydroxytryptamine creatinine sulfate monohydrate is an endogenous metabolite.
-
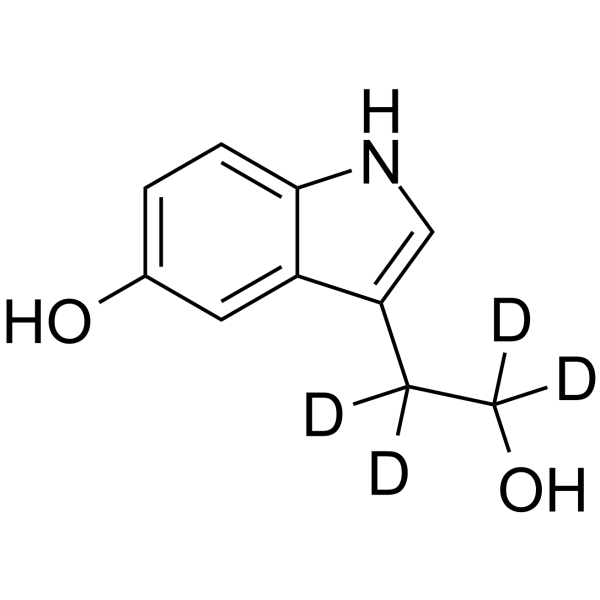
GC67951
5-Hydroxytryptophol-d4
-
GC31997
5-Lipoxygenase-In-1
5-Lipoxygenase-In-1 is a 5-Lipoxygenase inhibitor extracted from patent EP 331232 A2, table 4, compound example 4.10.
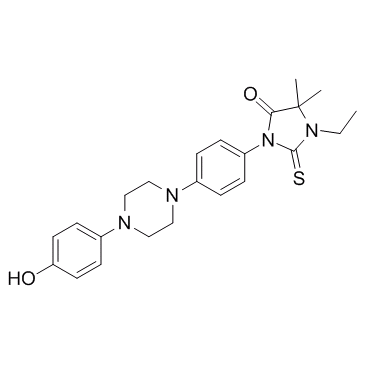
-
GC66031
5-LOX-IN-1
5-LOX-IN-1 (compound 2b) is an inhibitor of human 5-Lipoxygenase (5-LOX) with an IC50 value of 2.3 μM. 5-LOX-IN-1 can be used for the research of inflammation.
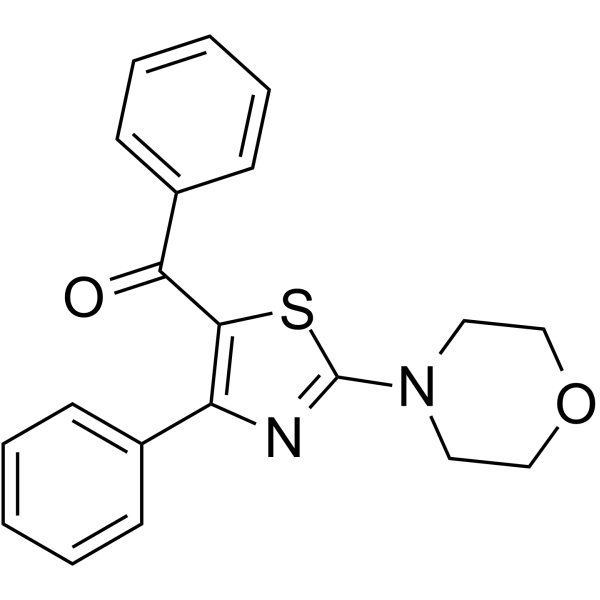
-
GC62811
5-Methoxy-5-oxopentanoic acid
5-Methoxy-5-oxopentanoic acid is an endogenous metabolite.
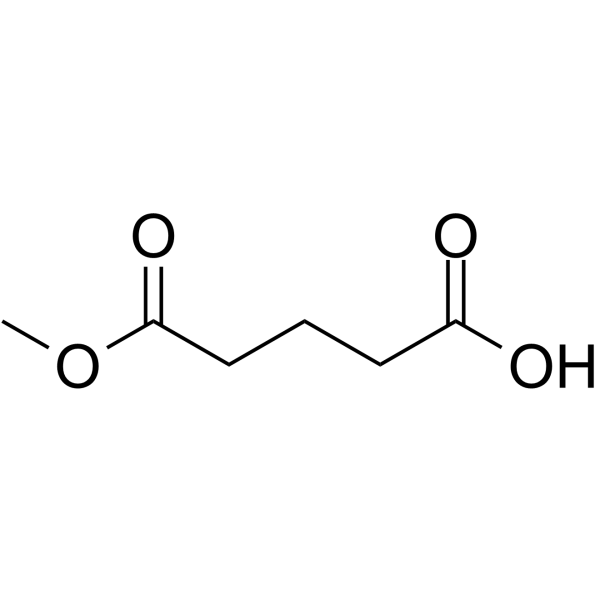
-
GC38058
5-Methoxy-DL-tryptophan
An active metabolite of tryptophan
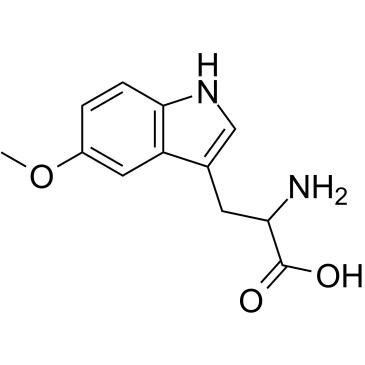
-
GC46078
5-Methoxyindole-3-acetic acid
A methoxyindole
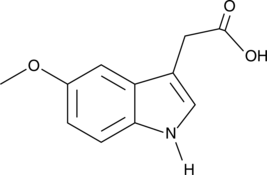
-
GC64182
5-Methoxytryptamine hydrochloride
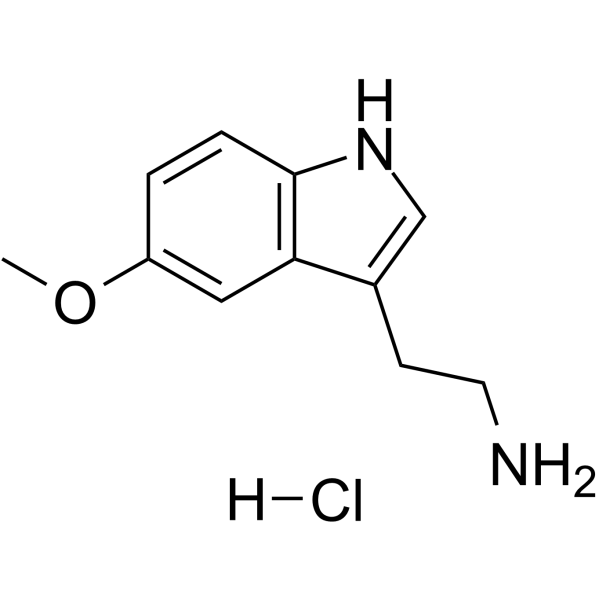
-
GC30712
5-Methoxytryptophol
A natural indole
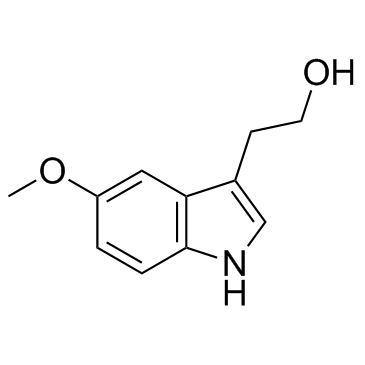
-
GC42562
5-Methyl-2'-deoxycytidine
5-Methyl-2'-deoxycytidine is a pyrimidine nucleoside that when incorporated into single-stranded DNA can act in cis to signal de novo DNA methylation.
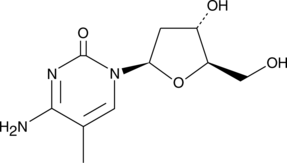
-
GC33526
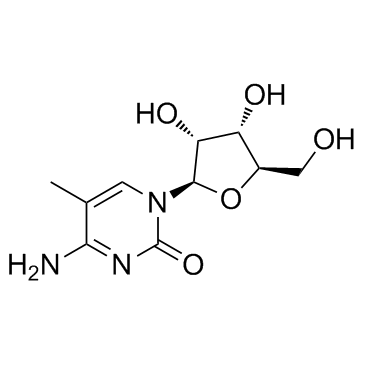
5-Methylcytidine
A modified nucleoside
-
GC35166
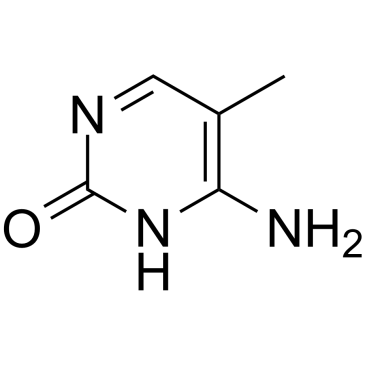
5-Methylcytosine
5-Methylcytosine is a well-characterized DNA modification, and is also predominantly in abundant non-coding RNAs in both prokaryotes and eukaryotes.
-
GC34881
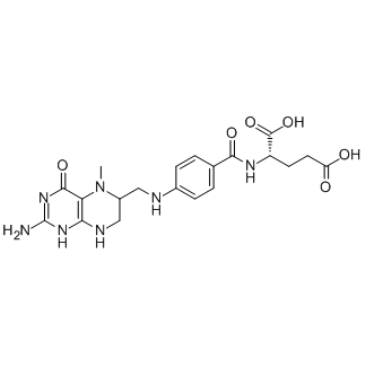
5-Methyltetrahydrofolic acid
5-Methyltetrahydrofolic acid (5-Methyl THF) is a biologically active form of folic acid.
-
GC33514
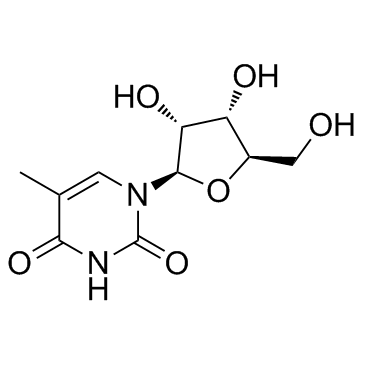
5-Methyluridine
A pyrimidine nucleoside
-
GC68228
5-Nitro-1,10-phenanthroline

-
GC35168
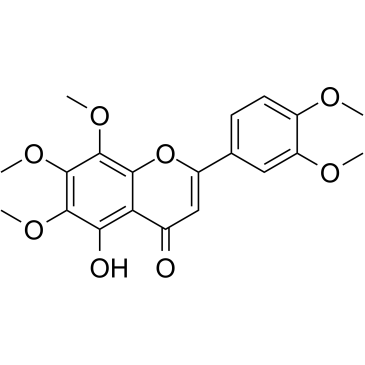
5-O-Demethylnobiletin
5-O-Demethylnobiletin (5-Demethylnobiletin), a polymethoxyflavone isolated from Sideritis tragoriganum, is a direct inhibition of 5-LOX (IC50=0.1 μM), without affecting the expression of COX-2.
-
GC41310
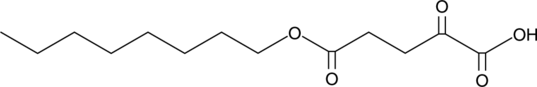
5-Octyl-α-ketoglutarate
In addition to its role in the Krebs cycle, α-ketoglutarate (2-oxoglutarate) has roles as a substrate or modulator of enzymes.
-
GC40380
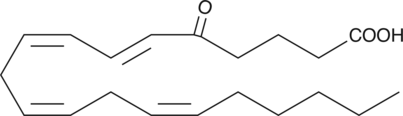
5-OxoETE
5-OxoETE is a polyunsaturated keto acid formed by the oxidation of 5-HETE in human neutrophils by a specific dehydrogenase.
-
GC41322
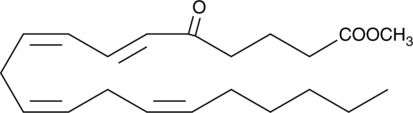
5-OxoETE methyl ester
5-OxoETE methyl ester is an esterified form of the polyunsaturated keto acid 5-oxoETE.
-
GC62814
5-Phenylvaleric acid
5-Phenylvaleric acid (5-Phenylpentanoic acid) is a pentanoic acid of bacterial origin, occasionally found in human biofluids.
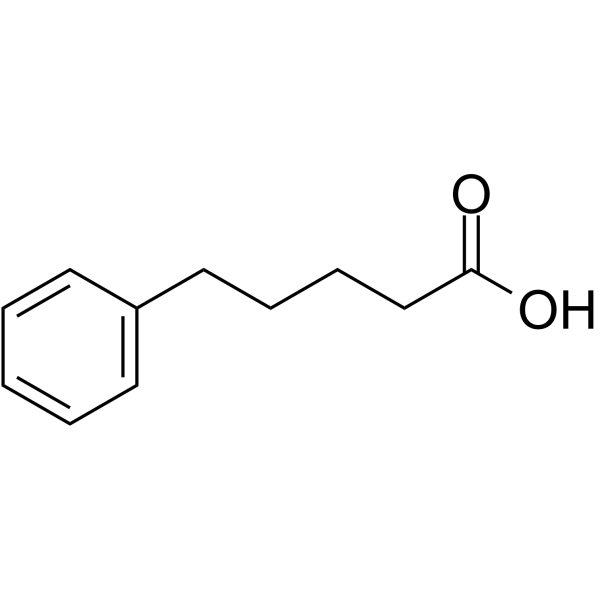
-
GC11101
5-R-Rivaroxaban
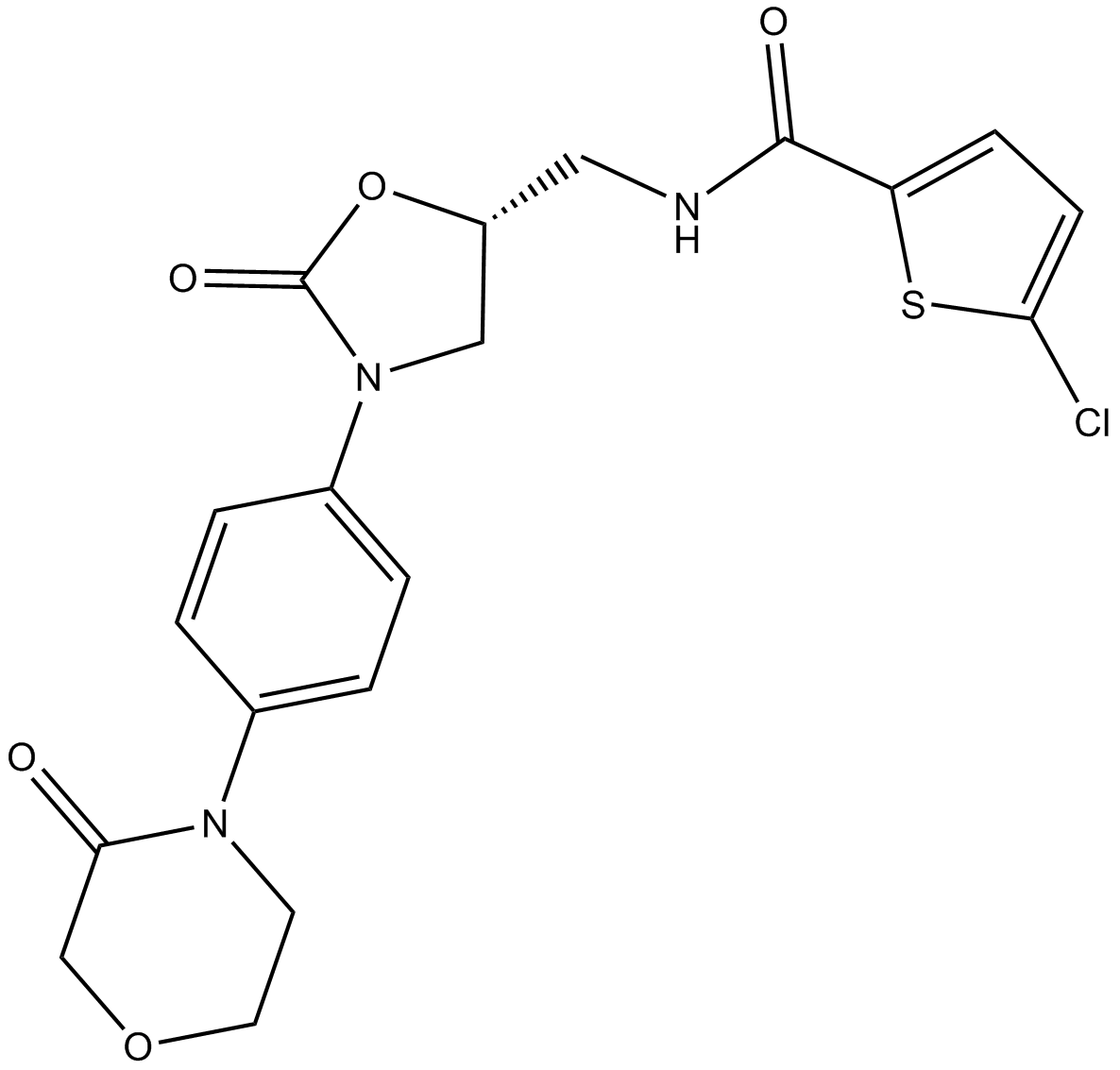
-
GC32410
5a-Pregnane-3,20-dione
A progesterone receptor agonist and progesterone metabolite
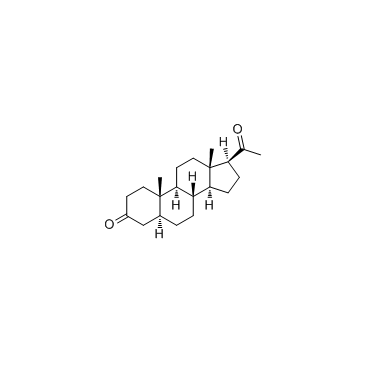
-
GC68374
5a-Pregnane-3,20-dione-d6
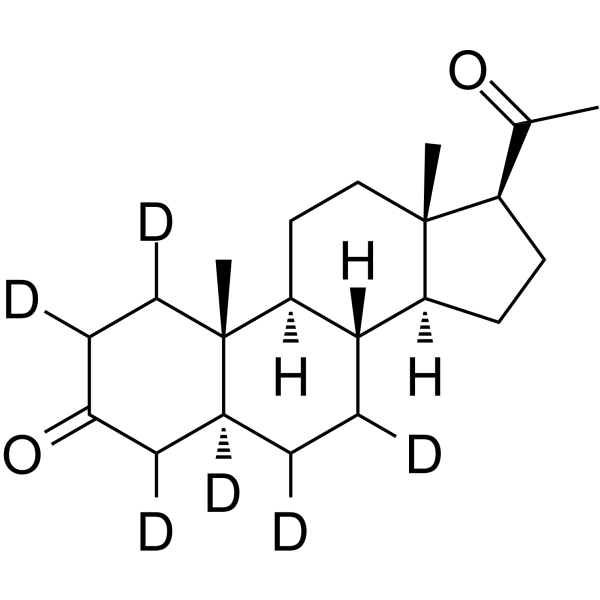
-
GC42586
6α-hydroxy Paclitaxel
6α-hydroxy Paclitaxel is a primary metabolite of the anticancer compound paclitaxel, produced by the action of the cytochrome P450 isoform CYP2C8.
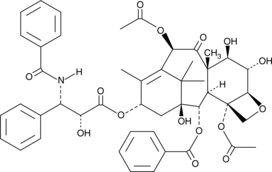
-
GC49676
6β-hydroxy Budesonide
A metabolite of budesonide

-
GC45969
6β-hydroxy Eplerenone
A major metabolite of eplerenone
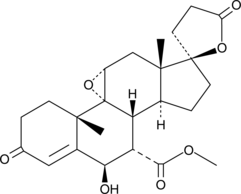
-
GC49629
6β-hydroxy Prednisolone
A metabolite of prednisolone
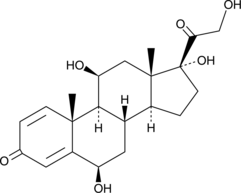
-
GC41424
6(S)-Lipoxin A4
The lipoxins are trihydroxy fatty acids containing a 7,9,11,13-conjugated tetraene.
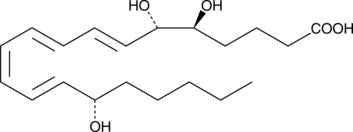
-
GC49749
6-Deoxypenciclovir
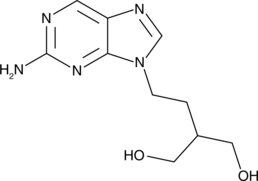
An inactive metabolite of famciclovir
-
GC41224
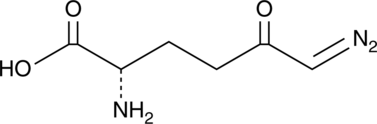
6-diazo-5-oxo-L-nor-Leucine
6-Diazo-5-oxo-L-nor-Leucine (DON) is a glutamine analog that inhibits glutaminases, a selective, mechanism-based inactivator of glutamine-using enzymes[1-3].
-
GC42578
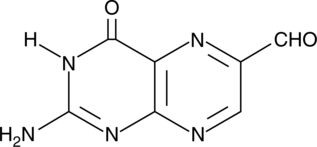
6-Formylpterin
Xanthine oxidase (XO) generates reactive oxygen species, including hydrogen peroxide (H2O2), as it oxidizes specific substrates in the presence of water and oxygen.
-
GC18632
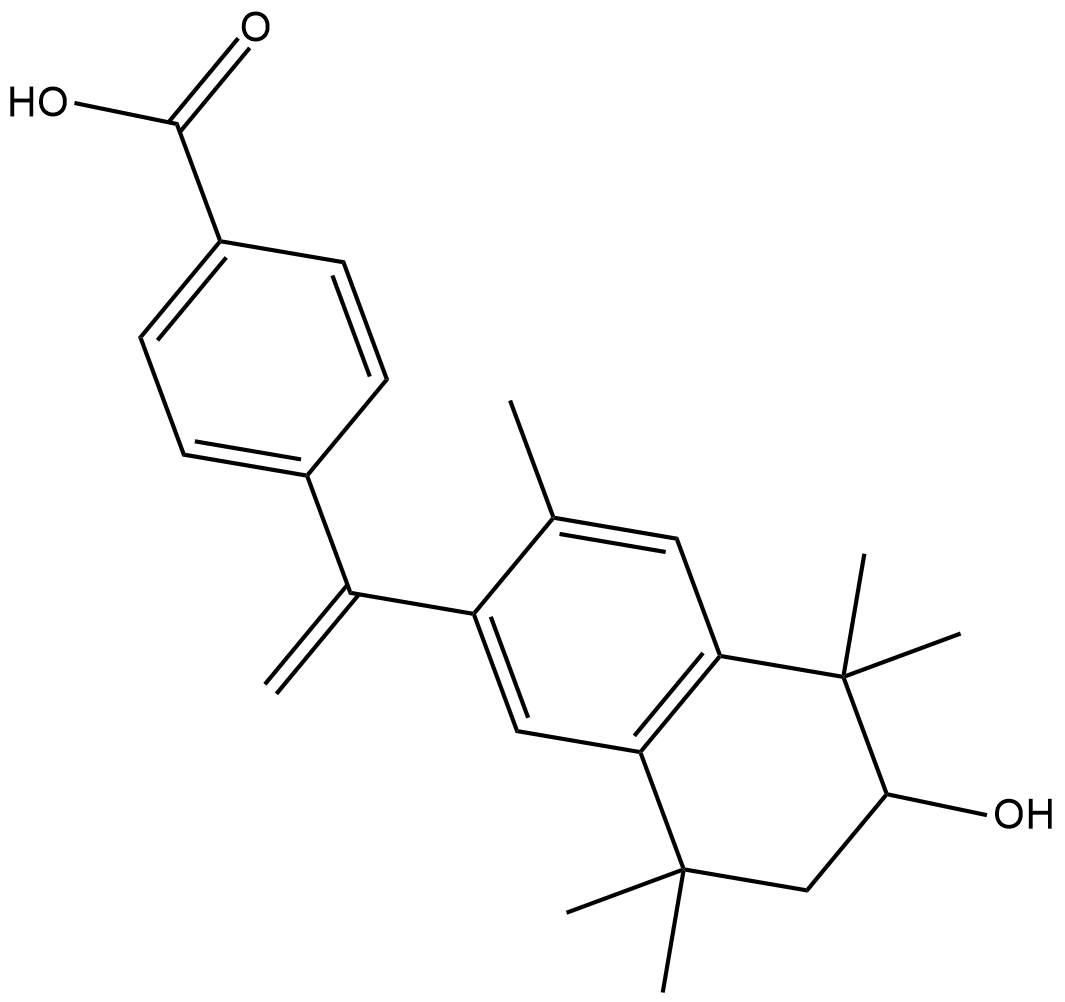
6-hydroxy Bexarotene
6-hydroxy Bexarotene is an oxidative metabolite of bexarotene , a high-affinity ligand for retinoid X receptors (RXRs).
-
GC42580
6-hydroxy Chlorzoxazone
6-hydroxy Chlorzoxazone is a metabolite of chlorzoxazone.
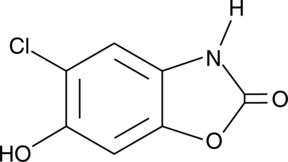
-
GC18219
6-hydroxy Warfarin
6-hydroxy Warfarin is a metabolite of (+)-warfarin , which is a weaker vitamin K antagonist than (-)-warfarin .
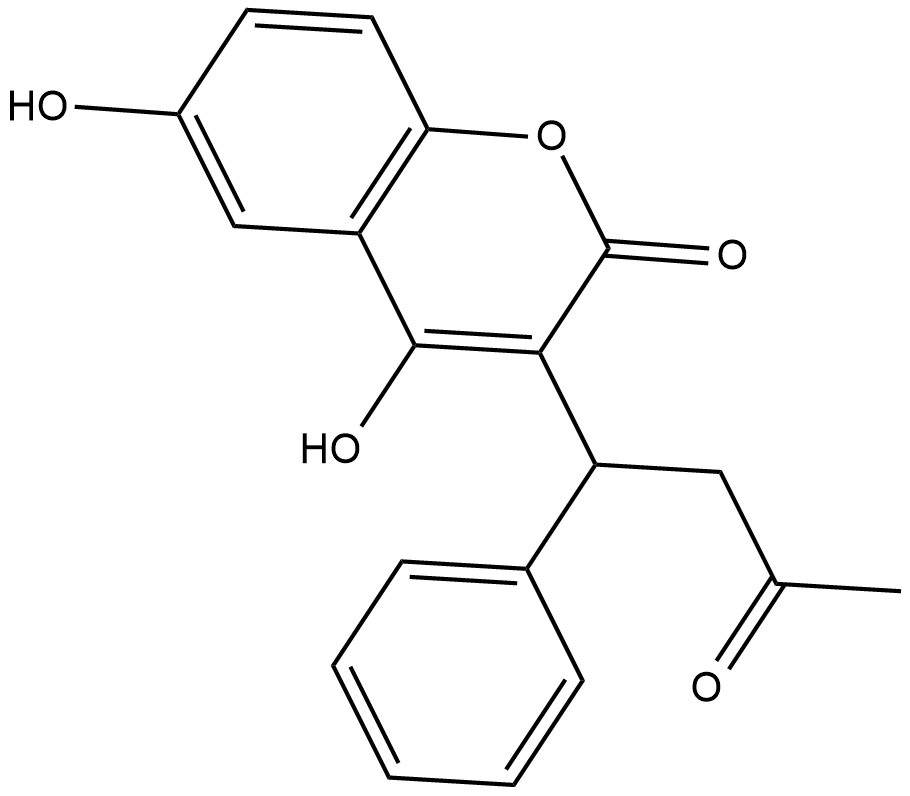
-
GC67988
6-Hydroxybenzbromarone
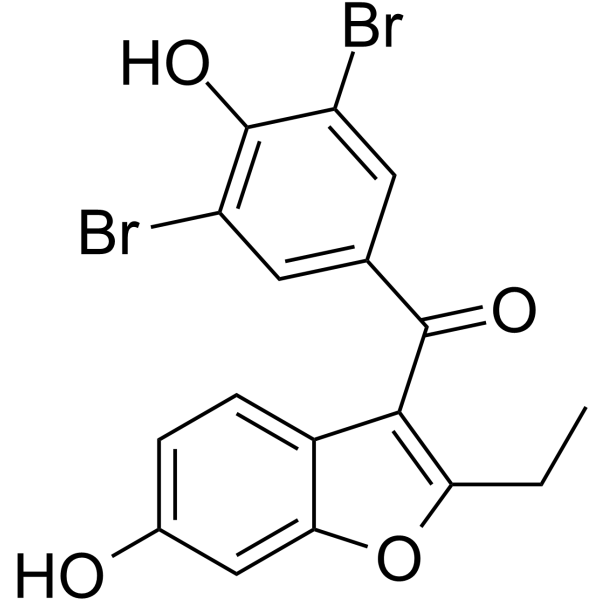
-
GC33704
6-Hydroxymelatonin
6-Hydroxymelatonin is a primary metabolic of Melatonin, which is metabolized by cytochrome P450 (CYP) 1A2.
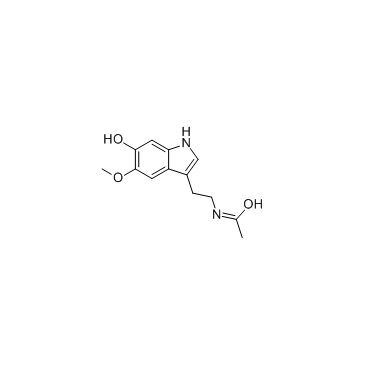
-
GC38278
6-Hydroxynicotinic acid
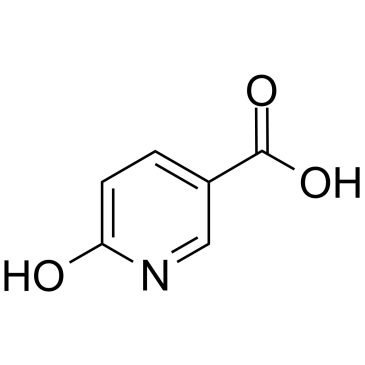
-
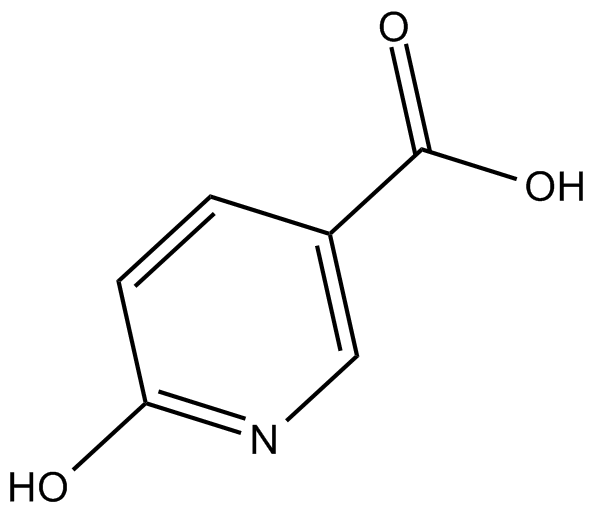
GC10067
6-Hydroxynicotinic acid
NULL
-
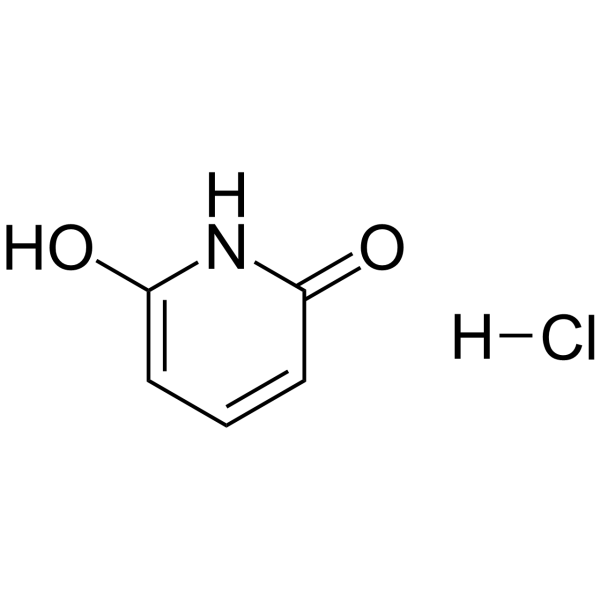
GC62816
6-Hydroxypyridin-2(1H)-one hydrochloride
6-Hydroxypyridin-2(1H)-one hydrochloride is an endogenous metabolite.
-
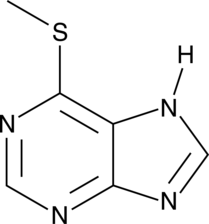
GC49235
6-Methylmercaptopurine
A metabolite of 6-mercaptopurine
-
GC49488
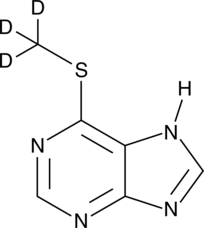
6-Methylmercaptopurine-d3
An internal standard for the quantification of 6-MMP
-
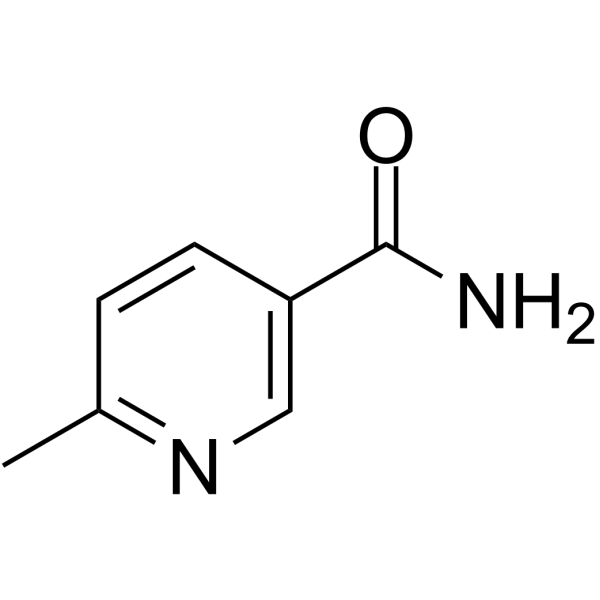
GC64741
6-Methylnicotinamide
6-Methylnicotinamide, a derivate of nicotinamide, is an endogenous metabolite.
-
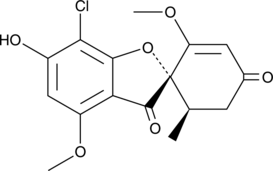
GC48721
6-O-Demethyl Griseofulvin
A metabolite of griseofulvin
-
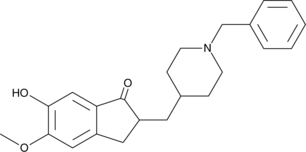
GC42584
6-O-desmethyl Donepezil
6-O-desmethyl Donepezil is an active metabolite of the acetylcholinesterase inhibitor donepezil.
-
GC65015
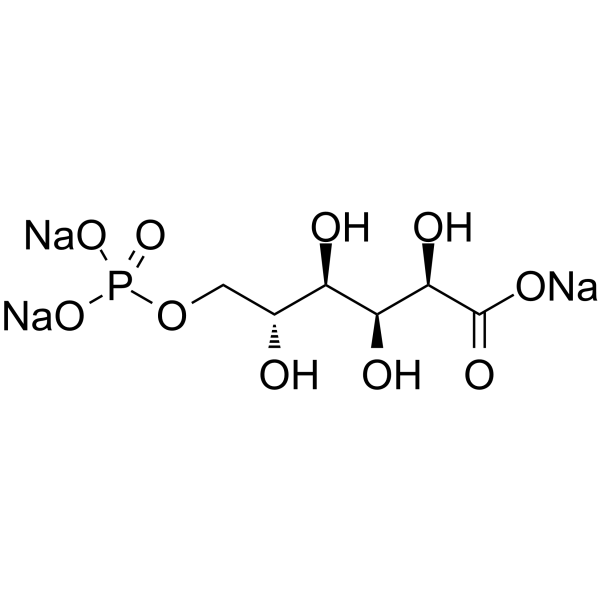
6-Phosphogluconic acid trisodium
6-Phosphogluconic acid trisodium is a potent and competitive phosphoglucose isomerase (PGI) inhibitor with Kis of 48 μM for glucose 6-phosphate and 42 μM for fructose 6-phosphate.
-
GC50613
673 A
ALDH1A inhibitor; depletes CD133+ cancer stem cells (CSC)
-
GC18335
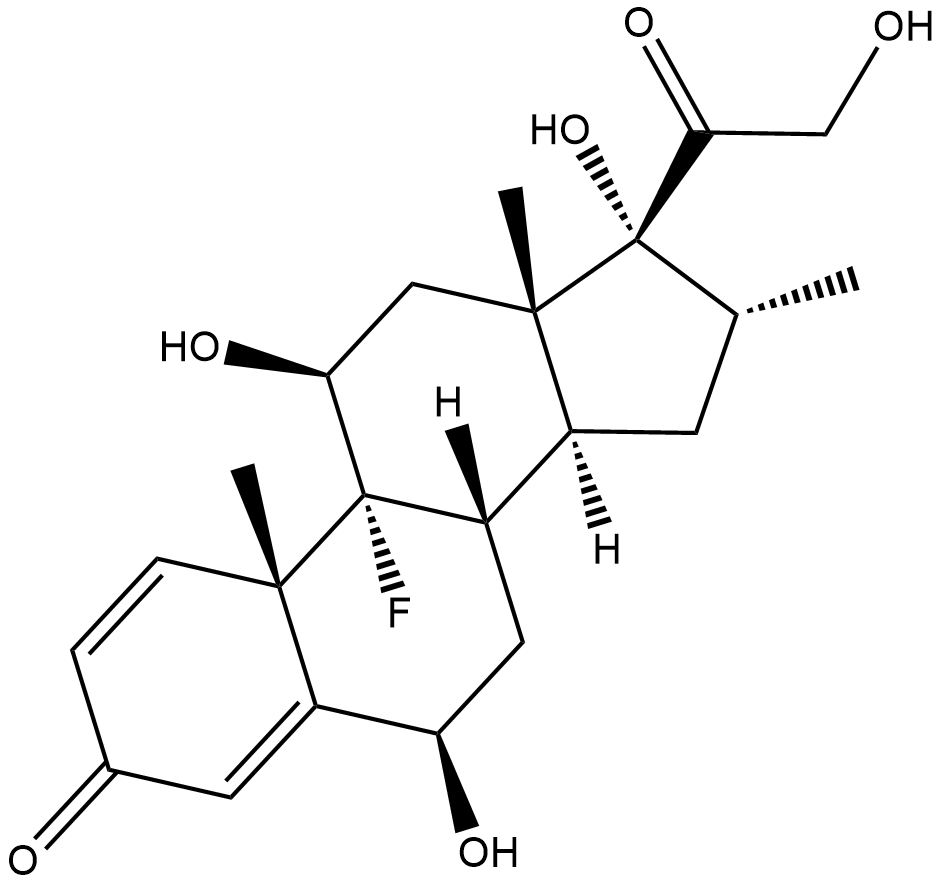
6β-hydroxy Dexamethasone
6β-hydroxy Dexamethasone is a metabolite of dexamethasone that is more hydrophilic than the parent compound.
-
GC40087
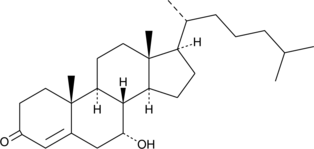
7α-hydroxy-4-Cholesten-3-one
7α-hydroxy-4-Cholesten-3-one is a metabolite of 7α-hydroxy cholesterol and an intermediate in the biosynthesis of bile acids.
-
GC49298
7α-Thiomethylspironolactone
A major metabolite of spironolactone
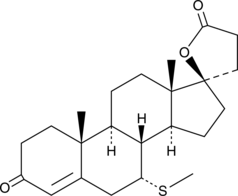
-
GC49391
7α-Thiospironolactone
An active metabolite of spironolactone
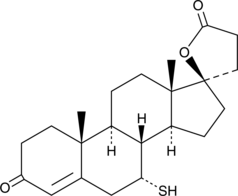
-
GC46732
7(S),17(S)-dihydroxy-8(E),10(Z),13(Z),15(E),19(Z)-Docosapentaenoic Acid
A metabolite of DPA with antiinflammatory properties
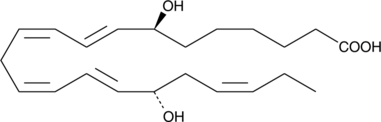
-
GC10533
7,8-dihydro-L-Biopterin
A precursor in the synthesis of BH4
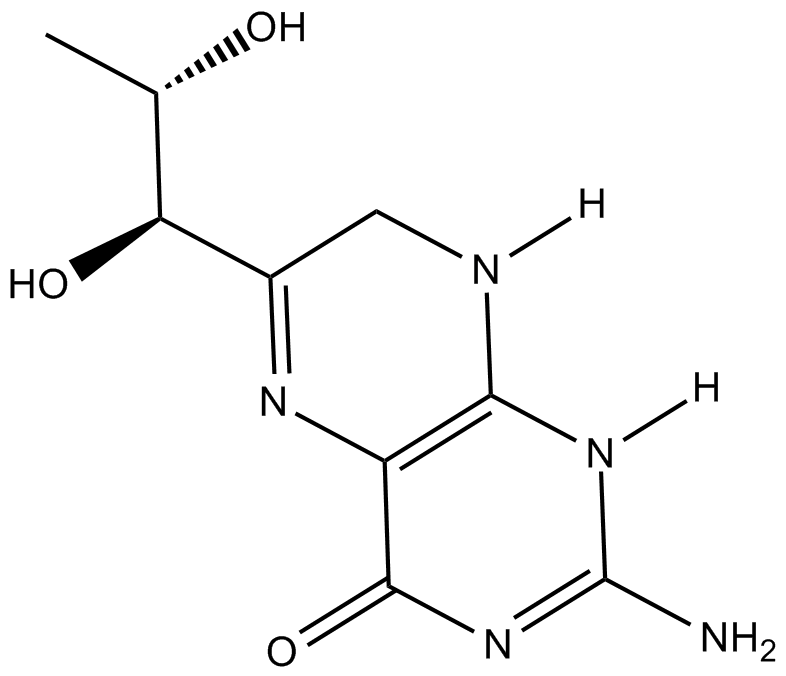
-
GC45673
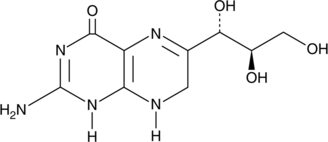
7,8-Dihydroneopterin
An antioxidant
-
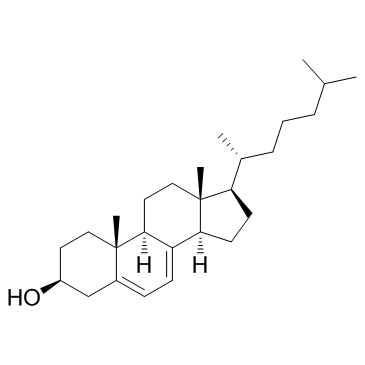
GC33450
7-Dehydrocholesterol
An immediate precursor to cholesterol
-
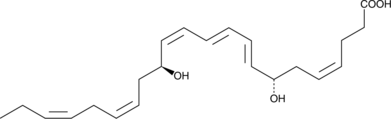
GC40978
7-epi Maresin 1
7-epi Maresin 1 is the inactive 7(S) epimer of Maresin 1, which contains a 7(R) hydroxyl group.
-
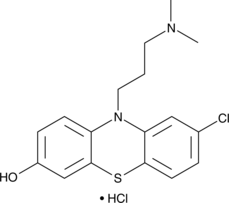
GC48649
7-hydroxy Chlorpromazine (hydrochloride)
An active metabolite of chlorpromazine
-
GC42606
7-hydroxy Coumarin Glucuronide (sodium salt)
7-hydroxy Coumarin glucuronide is a 7-hydroxy coumarin phase II metabolite that can be used as a standard for the analysis of 7-hydroxy coumarin metabolism.
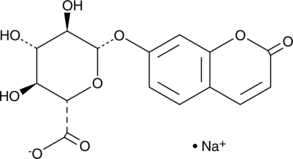
-
GC42607
7-hydroxy Coumarin sulfate (potassium salt)
7-hydroxy Coumarin sulfate is a phase II metabolite of coumarin that can be used as an internal standard for the analysis of 7-hydroxy coumarin metabolism using GC- or LC-MS.
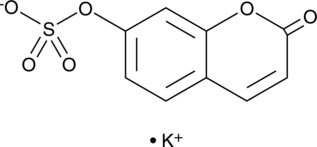
-
GC49748
7-hydroxy Etodolac
An inactive metabolite of etodolac
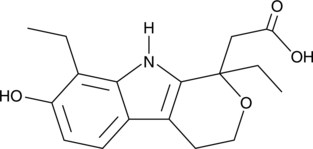
-
GC49051
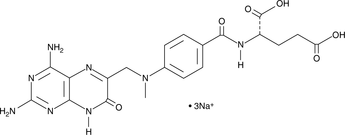
7-hydroxy Methotrexate
A metabolite of methotrexate
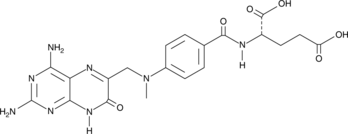
-
GC42608
7-hydroxy Methotrexate (sodium salt)
7-hydroxy Methotrexate (7-hydroxy MTX) is a phase I metabolite of MTX, which is converted by hepatic aldehyde oxidases.